- EN - English
- CN - 中文
Isolation and Long-term Cultivation of Mouse Alveolar Macrophages
小鼠肺泡巨噬细胞的分离与长期培养
发布: 2019年07月20日第9卷第14期 DOI: 10.21769/BioProtoc.3302 浏览次数: 16991
评审: Ruth A. FranklinJason A. NeidlemanPedro Escoll

相关实验方案
来自骨髓增生性肿瘤患者的造血祖细胞的血小板生成素不依赖性巨核细胞分化
Chloe A. L. Thompson-Peach [...] Daniel Thomas
2023年01月20日 2360 阅读
Abstract
Alveolar macrophages (AM) are tissue-resident macrophages that colonize the lung around birth and can self-maintain long-term in an adult organism without contribution of monocytes. AM are located in the pulmonary alveoli and can be harvested by washing the lungs using the method of bronchoalveolar lavage (BAL). Here, we compared different conditions of BAL to obtain high yields of murine AM for in vitro culture and expansion of AM. In addition, we describe specific culture conditions, under which AM proliferate long-term in liquid culture in the presence of granulocyte-macrophage colony-stimulating factor. This method can be used to obtain large numbers of AM for in vivo transplantation or for in vitro experiments with primary mouse macrophages.
Keywords: Macrophage (巨噬细胞)Background
AM are resident tissue macrophages of the lungs with critical importance for immune regulation and surfactant homeostasis (Kopf et al., 2015). Due to their localization in the airspace of the alveoli, AM are directly exposed to inhaled air and pathogens or other aerosolized particles. Consequently, AM play a crucial role in the initiation or suppression of inflammatory responses and are the subject of investigation in numerous studies that explore mechanisms of pulmonary diseases (Hodge et al., 2007; Sun and Metzger, 2008; Happle et al., 2014; Schneider et al., 2014a; Machiels et al., 2017; Yu et al., 2017). Interestingly, AM in the mouse originate from fetal monocytes and are able to self-maintain their numbers in vivo without contribution of bone-marrow-derived monocytes under steady state conditions (Guilliams et al., 2013; Hashimoto et al., 2013). AM are unique in that they reside outside of the body surface and are directly exposed to the external environment. They can therefore be isolated with minimal tissue disturbance using bronchoalveolar lavage (BAL). We previously demonstrated that the self-renewal property of AM harvested by BAL is maintained in culture by growing AM long-term in liquid media or serially re-plating AM in semi-solid media (Soucie et al., 2016; Imperatore et al., 2017). Here, we describe the methodological advancements in BAL and specific culturing conditions optimized for long-term culture and high AM yields.
To obtain sufficient quality and numbers of AM, we tested different cell harvesting conditions and developed a culture method that allows long-term maintenance of AM in vitro. Our BAL method was based on earlier studies using pre-warmed PBS and EDTA for detaching AM from lung alveoli (Steele et al., 2003; Zhang et al., 2008). Additionally, serum was added for cellular protection during the isolation period. Importantly, both omitting EDTA in the lavage buffer and using pre-cooled PBS for lavage resulted in lower AM yield in our hands. Our comparisons demonstrated that the variable with the largest effect on yield was the temperature of the BAL buffer (Figure 1). Whereas many earlier studies obtain BAL cell numbers below 2 x 105 per wild-type mouse (Table 1), our comparative analysis indicated that BAL cell numbers can be considerably increased by these optimizations. We generally obtained up to 5 x 105-7 x 105 live (Trypan-Blue-negative) cells per mouse using this method (Figure 1). FACS analysis revealed that more than 98% of cells were alive at the time of recording using the Zombie Violet Fixable Viability Kit, independent of whether the BAL was performed with 4 °C PBS or 37 °C PBS/EDTA/FBS. Our experience has shown that when performing dozens of BALs on the same day long waiting times on ice will result in higher cell death unless low amounts of FBS are added to the BAL buffer (typically 0.5%, although we have good experience with up to 2%). 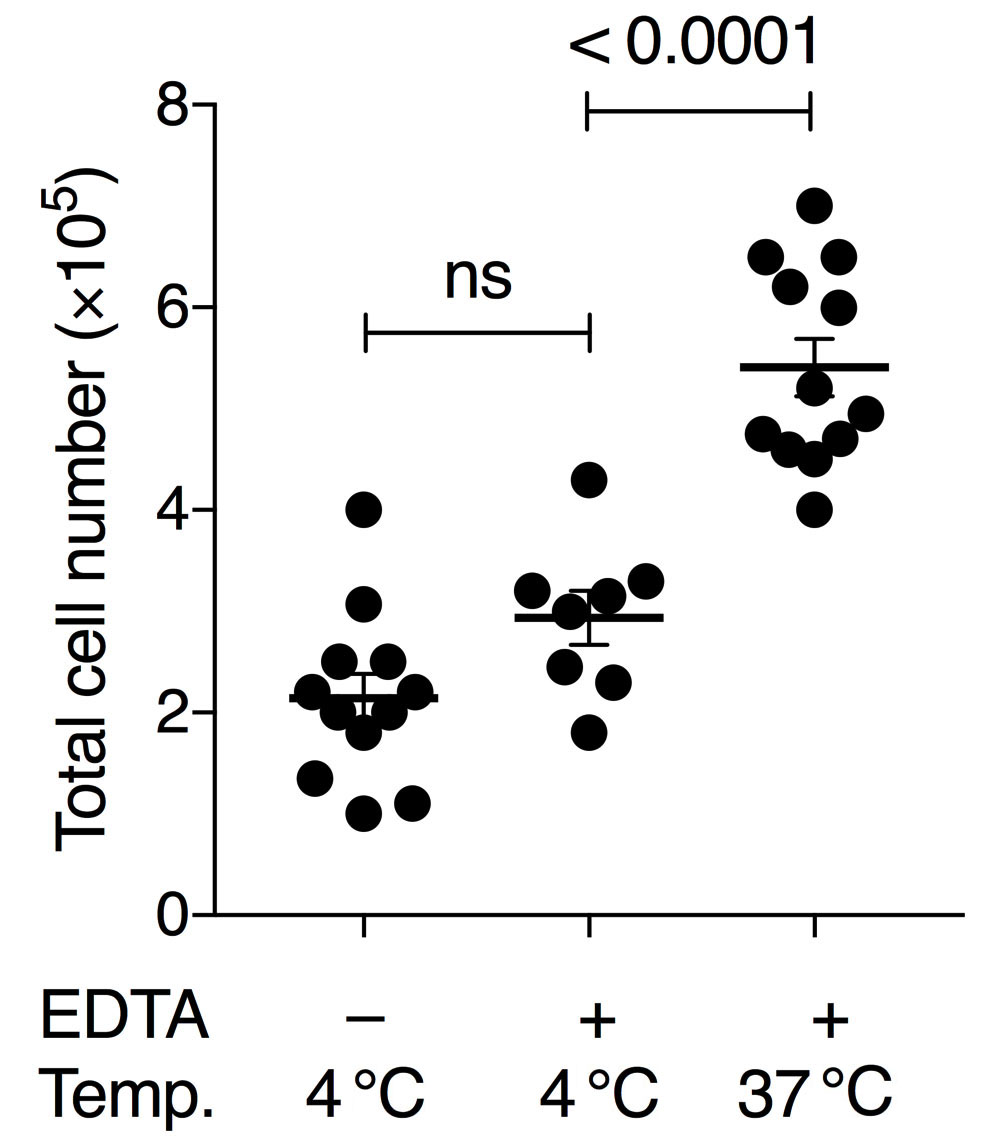
Figure 1. Comparison of BAL conditions using pre-cooled or pre-warmed PBS with or without 2 mM EDTA. Either 4 °C PBS without EDTA, 4 °C PBS with 2 mM EDTA and 0.5% FBS, or 37 °C PBS with 2 mM EDTA and 0.5% FBS was used. Numbers show the total amount of living cells (Trypan-Blue-negative) per BAL treatment per mouse. Each symbol denotes the mean cell count of 3 technical replicates of an individual mouse; horizontal lines indicate the mean, error bars show standard error of the mean (SEM); one-way ANOVA with Tukey’s multiple comparisons test; ns, non-significant.
Table 1. Comparison of the efficiency of different BAL protocols. Total cell number in BAL fluid obtained in cited studies. Only counts from WT animals (or comparable conditions, such as control-treated WT animals) were considered. WT denotes wild-type mouse.
Increased efficiency in harvesting BAL cells is advantageous for in vivo reconstitution of multiple recipient animals such as strains devoid of endogenous AM, such as GM-CSF-receptor-deficient mice (Guilliams et al., 2013; van de Laar et al., 2016). High starting cell numbers and viability of harvested cells will accelerate establishment of long-term AM cultures and improve cellular yield. We also noticed several culture conditions outlined in the detailed protocol that affect the quality, yield, doubling time and durability of the cultures. In order to avoid cell activation or death, several parameters need to be controlled. Firstly, we used exclusively sterile supplies and applied sterile handling techniques to avoid activation of AM. Secondly, we use non-treated plastic ware (not tissue-culture treated plates or dishes) and a gentle detachment protocol. Together, these technical improvements will be helpful for starting and maintaining a long-term AM culture.
Biochemical and genetic manipulations of macrophages that require a large number of cells could so far only be done in cell lines, such as RAW 264.7 or J774A.1 cells (Ralph and Nakoinz, 1975; Raschke et al., 1978), oncogene-transformed cells, for example Myc (Baumbach et al., 1986; Baumbach et al., 1987) or SV40 transformed macrophages like IC-21 (Walker and Demus, 1975) or MH-S cells (Mbawuike and Herscowitz, 1989), macrophages differentiated from progenitors (Zhang et al., 2008; Fejer et al., 2013) or non-transformed but genetically modified macrophages, such as Maf-DKO macrophages (Aziz et al., 2009). The ability to obtain large numbers of normal unmodified AM in culture allows such experiments in primary resident macrophages. Our studies on macrophage self-renewal mechanisms serve as an example (Soucie et al., 2016; Imperatore et al., 2017).
Materials and Reagents
- 15-ml conical tubes (Corning, catalog number: 352196)
- Bottle-top vacuum filter with 0.22 μm membrane (Corning, catalog number: 431161)
- Plastic storage bottle (Corning, catalog number: 430281)
- 70-μm sterile cell strainer (BD, catalog number: 340633)
- 1-ml syringe (Braun, catalog number: 9161406V)
- 18-G cannula (Braun, catalog number: 4667123)
- Petri dish 94/16 mm (Greiner Bio-one, catalog number: 633181)
- Non-treated 6-well plate (NuncTM, catalog number: 150239)
- C57BL/6 mice (aged 6-10 weeks)
- PBS, pH 7.2 (Thermo Fisher Scientific, GibcoTM, catalog number: 20012019)
- EDTA stock solution (e.g., 0.5 M, pH 8.0)
- Hemolysis buffer (self-made or commercial, e.g., Morphisto, catalog number: 12146)
- Trypan Blue solution 0.4% (Sigma-Aldrich, catalog number: T8154)
- RPMI 1640 Medium, no glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 31870025)
- Fetal bovine serum (Testing of different batches is recommended)
- Gentamicin sulphate 50 mg/ml in aqueous solution (Lonza, catalog number: BE02-012E)
- Penicillin-Streptomycin (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122)
- Sodium Pyruvate (100 mM) (Thermo Fisher Scientific, GibcoTM, catalog number: 11360070)
- GlutaMAXTM Supplement (Thermo Fisher Scientific, GibcoTM, catalog number: 35050038)
- Conditioned medium from J558L cell line transfected with murine GM-CSF cDNA as a source for GM-CSF (Zal et al., 1994; Stockinger et al., 1996; Rayasam, 2015)
- ESGRO Complete Accutase (Merck, catalog number: SF006)
- EGTA stock solution (e.g., 0.5 M, pH 8.0)
- UltraComp eBeadsTM Compensation Beads (Thermo Fisher Scientific, Invitrogen, catalog number: 01-2222-41)
- Zombie Violet Fixable Viability Kit (BioLegend, catalog number: 423113)
- FACS antibodies (as indicated in Table 2)
- BAL buffer (see Recipes)
- Complete medium (see Recipes)
- AM culture medium (see Recipes)
- Detachment medium (see Recipes)
Equipment
- Pipettes
- Mouse dissection tools (scissors, forceps)
- Water bath set to 37 °C
- Refrigerated benchtop centrifuge for spinning conical tubes
- Hemocytometer (Roth, catalog number: T729.1)
- Incubator (37 °C, 5% CO2)
- Inverse microscope
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Busch, C. J., Favret, J., Geirsdóttir, L., Molawi, K. and Sieweke, M. H. (2019). Isolation and Long-term Cultivation of Mouse Alveolar Macrophages. Bio-protocol 9(14): e3302. DOI: 10.21769/BioProtoc.3302.
分类
免疫学 > 免疫细胞分离 > 巨噬细胞
免疫学 > 免疫细胞分离 > 维持和分化
细胞生物学 > 细胞分离和培养 > 细胞分离 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link