- EN - English
- CN - 中文
Isoelectric Focusing to Quantify Rhodopsin Phosphorylation in Mouse Retina
等电聚焦用于小鼠视网膜中磷酸化视紫红质的定量分析
发布: 2019年07月20日第9卷第14期 DOI: 10.21769/BioProtoc.3300 浏览次数: 6866
评审: Oneil G. BhalalaAnonymous reviewer(s)
Abstract
Rhodopsin is a G-protein coupled receptor (GPCR) that mediates vision under dim light. Upon light exposure, rhodopsin is phosphorylated at multiple serine and threonine sites at its carboxyl-terminus by rhodopsin kinase (GRK1). This, in turn, reduces its ability to activate the visual G-protein transducin. Binding of light-activated, phosphorylated rhodopsin by arrestin (ARR1) fully terminates the catalytic activity of rhodopsin. Quantification of the levels of the differentially phosphorylated rhodopsin species provides definitive information about the role of phosphorylated rhodopsin in visual functions. Isoelectric Focusing (IEF) is a technique which is used to separate ampholytic components, such as proteins, based on their isoelectric point (pI). It is a useful technique used to distinguish protein isoforms and post-translational modifications such as phosphorylation, glycosylation, deamination, and acetylation, due to their effects on the protein’s pI. Isoelectric Focusing can provide high resolution of differentially phosphorylated forms of a protein. Though other techniques such as kinase activity assays, phospho-specific antibodies, western blot, enzyme-linked immunosorbent assays (ELISA), radiolabeling and mass spectrometry are used to detect and quantify protein phosphorylation, IEF is a simple and cost-effective method to quantify rhodopsin phosphorylation, as it can readily detect individual phosphorylated forms.
Here we provide a detailed protocol for determining phosphorylated rhodopsin species using the Isoelectric Focusing technique.
Background
Rhodopsin is a seven-helix transmembrane protein covalently linked to a chromophore, 11-cis-retinal, which acts as a powerful antagonist, holding rhodopsin in its inactive state. Photon absorption isomerizes the retinal from cis to trans which then triggers conformational changes in the protein moiety into a catalytically active form. Activated rhodopsin facilitates GTP-GDP exchange in the visual G-protein transducin. Transducin-GTP binds and releases the inhibitory constraint of the gamma subunit on phosphodiesterase 6 (PDE6), which then hydrolyzes the diffusible second messenger cGMP, leading to closure of cation cGMP gated channels localized in the plasma membrane. This collective of reactions, called the phototransduction cascade, provides the first steps of vision and is one of the best characterized G-protein signaling pathways (Molday and Moritz, 2015).
Like other GPCRs, rapid quenching of receptor activity depends on phosphorylation by a receptor kinase and subsequent binding of arrestin. During rhodopsin deactivation, rhodopsin kinase (GRK1) sequentially phosphorylates six serine/threonine residues contained within residues 334-343 near the C-terminus of mouse rhodopsin: SATASKTETS. These multiple phosphorylation sites confer reproducibility of the rod’s quantal response to single photon absorption (Mendez et al., 2000; Doan et al., 2006; Azevedo et al., 2015) which is essential for our ability to see under dim light conditions when photons are sparse. In the absence of ARR1, rhodopsin phosphorylation alone decreases the efficiency of transducin activation (Xu et al., 1997). Even single and double phosphorylations can decrease rhodopsin’s catalytic activity by half (Mendez et al., 2000). Therefore, a reliable methodology to quantify rhodopsin phosphorylation will provide important information on how this common post-translational modification affects phototransduction, and thus visual performance.
Traditionally, the most common method used to detect protein phosphorylation involved the use of radiolabeling. Phosphorylation of rhodopsin by GRK1 has been determined using radiolabeled [γ-32P] ATP (Zhang et al., 1997). Greene et al. (1995) have also shown kinetic and structural analysis of the phosphorylation of rhodopsin by protein kinase C. However, the limitations of the radiolabeling method include sample variability and an inability to quantify pre-bound unlabeled phosphate. Reverse phase column chromatography has been used to separate phospho- and non-phospho mouse rhodopsin peptides, and to study the kinetics of phosphorylation/dephosphorylation (Ohguro and Palczewski, 1995). Phospho-specific antibodies are also routinely used to analyze key targets in normal and disease states. Though useful for initial studies, phospho-specific antibodies fail to detect unphosphorylated species; hence stoichiometric measurements cannot be made. Production of quality phospho-specific antibodies is time-consuming, costly, and also requires prior knowledge of specific phosphorylation sites (Arur and Schedl, 2014).
Mass spectrometry has become a highly sensitive nonradioactive method to analyze protein phosphorylation. Electrospray mass spectrometry has been used to identify sequential phosphorylation of rhodopsin at multiple sites in vitro (Ohguro et al., 1993) and in vivo (Kennedy et al., 2001; Lee et al., 2002). However, major challenges in analyzing protein phosphorylation using mass spectrometry include access to the instrumentation and the possible high cost associated with using the instruments through core facilities. Additionally, mass accuracy of post-translational modifications depends on the resolution of the mass spectrometer used. Some of the intrinsic limitations are: a) mass spectrometry is sensitive in a particular m/z (mass-to-charge ratio) range; b) protease cleavage efficiencies vary for different proteins and different domains of same protein; and c) cleaved peptides have different ionization and detection efficiencies (Kim et al., 2016).
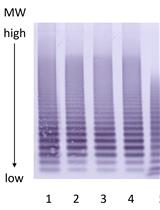
Isoelectric Focusing (IEF) is a high-resolution technique used for separating charged molecules, usually proteins or peptides, on the basis of their isoelectric point (pI) within a continuous pH gradient. pI is defined as the pH at which the molecule has no charge. Separation is achieved by initially establishing a stable pH gradient along the length of the gel using ampholytes. The sample components then travel through the pH gradient under an electric field until their individual charges reach zero. Proteins move towards the electrode with an opposite charge. The gradient is set up so that negatively charged molecules migrate towards decreasing pH, whereas positively charged molecules move towards increasing pH. For this technique, a low concentration polyacrylamide or agarose gel is used as it is only a stabilizing medium and does not participate in the sample separation. IEF can readily detect individual phosphorylated forms, as every additional phosphate increases the negative charge on rhodopsin protein, and hence causes a shift in pI (Berry et al., 2016). Here we describe a simple and cost-effective method to determine phosphorylated rhodopsin species using Isoelectric Focusing (IEF) in a horizontal gel format followed by immunoblot detection.
Materials and Reagents
- 2.0 ml sterile round bottom microfuge tubes (USA Scientific, catalog number: 1620-2700)
- 0.5 ml sterile microfuge tubes (USA Scientific, catalog number: 1605-0000)
- 10 μl Sterile pipette tips (USA Scientific, catalog number: 1111-3810)
- 200 μl Sterile pipette tips (USA Scientific, catalog number: 1111-1810)
- 1000 μl Sterile pipette tips (USA Scientific, catalog number: 1111-2821)
- 25 ml Serological pipettes (USA Scientific, catalog number: 1072-5410)
- 15 ml sterile conical centrifuge tubes (VWR International, catalog number: 10025-686)
- 50 ml sterile conical centrifuge tubes (VWR International, catalog number: 10025-698)
- Pipettes (Research–includes 3-pack [2-20 μl, 20-200 μl, 100-1,000 μl], Eppendorf, catalog number: EPPR3OP2)
- Nitrocellulose membrane (Sigma-Aldrich, catalog number: GE10600002)
- Blotting paper (Thermo Fisher Scientific, catalog number: 88600)
- Aluminum foil (VWR International, catalog number: 89107-724)
- 3 cm plastic Petri dish (Thermo Fisher Scientific, catalog number: 150318)
- Saran wrap (VWR International, catalog number: 89136-664)
- Ames’ medium (Sigma-Aldrich, catalog number: A1420-10X1L)
- Acrylamide ReadySol IEF 40% (GE Healthcare, catalog number: 17-1310-01)
- 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Omnipur, catalog number: 5320)
- Sodium fluoride (NaF) (Fischer Scientific, catalog number: S299-100)
- Adenosine (Sigma-Aldrich, catalog number: A-4036)
- Phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P-7626)
- Ethylenediaminetetraacetic acid (EDTA) (VWR, catalog number: BDH4616)
- Roche protease inhibitors (Sigma-Aldrich, catalog number: 11873580001)
- Milli-Q water
- Liquid nitrogen
- 95% O2 /5% CO2 gas cylinder
- Magnesium chloride (MgCl2) (EMD, catalog number: MX0045-1)
- Bovine Serum Albumin (BSA)-Fatty acid free (Sigma-Aldrich, catalog number: A-8806)
- Sodium chloride (NaCl) (EMD, catalog number: SX0420-3)
- Tris-HCl (Sigma-Aldrich, catalog number: T3253)
- Tween-20 (Sigma-Aldrich, catalog number: P1379)
- Nonfat-dried milk (Sigma-Aldrich, catalog number: M7409)
- n-dodecyl-β-D-maltoside (DDM) (Avanti Polar Lipids, catalog number: 850520)
- 11-cis-retinal (National Eye Institute) or 9-cis-retinal (Sigma-Aldrich, catalog number: R5754)
- Glycerol (Sigma-Aldrich, catalog number: G5516-1L)
- Glutamic acid (Sigma-Aldrich, catalog number: G-1251)
- Sodium hydroxide (EMD, catalog number: SX0593-3)
- Dithiothreitol (DTT) (Research Products International Corp [RPI], catalog number: D11000-1000)
- Mineral oil (Bio-Rad, catalog number: 1632129)
- Ammonium persulfate (Sigma-Aldrich, catalog number: A3678)
- TEMED (Sigma-Aldrich, catalog number: T9281)
- Rhodopsin antibody RET-P1 (Santa Cruz Biotechnology, catalog number: sc-57433)
- Peroxidase labeled anti-mouse IgG (Vector Laboratories, catalog number: PI-2000)
- Pharmalyte 2.5-5 for IEF (GE Healthcare, catalog number: 17-0451-01)
- Pharmalyte 5-8 for IEF (GE Healthcare, catalog number: 17-0453-01)
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S7907)
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655)
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541)
- Absolute ethanol (Sigma-Aldrich, catalog number: E7023)
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761)
- ECL western blotting detection reagents (GE Healthcare, catalog number: RPN2209)
- Buffer A (Homogenization buffer) (see Recipes)
- Buffer B (Regeneration buffer) (see Recipes)
- Buffer C (Solubilization buffer) (see Recipes)
- 11-cis-retinal stock (see Recipes)
- IEF Gel solution (see Recipes)
- 1x Phosphate buffered saline (PBS) (see Recipes)
- 1x Tris-buffered saline with Tween-20 (TBST) (see Recipes)
Equipment
- Photostimulator: A light bench for photostimulation of the retina
This device (Figure 1) consists of a tungsten-halogen light source, neutral density filters, interference filters, and other optics necessary to focus 500 nm light of uniform intensity on a spot roughly 5 mm in diameter, where a Petri dish containing the retina is placed. The intensity, duration and wavelength of light is chosen so as to bleach (photoactivate) a specified fraction of rhodopsin.
The components that comprise the optical system used for bleaching were all purchased from Thorlabs. Thorlabs components are modular and can be used in a variety of combinations to align all of the optical components. Intense white light is provided by a 6V 100 W tungsten-halogen lamp of the sort normally connected to laboratory light microscopes. The light source used in the Figure 1 (in-house setup) was taken from a discarded Zeiss microscope, but an illuminator from any other microscope manufacturer will serve the purpose as well. The components of the bleaching apparatus are mounted in the light path on an aluminum plate (Thorlabs; MB1218), which has an array of ¼”-20 threaded holes for affixing the components. The components are lined up in a linear fashion to allow adjustment of wavelength and intensity of the bleaching beam. First in line is a filter wheel (Thorlabs; CFW6) with positions for 5 one-inch diameter neutral density filters. The following neutral density filters are used:
NE05B-A 25.0 mm diameter absorptive ND filter ND = 0.5
NE10B-A 25.0 mm diameter absorptive ND filter ND = 1.0
NE20B-A 25.0 mm diameter absorptive ND filter ND = 2.0
NE30B-A 25.0 mm diameter absorptive ND filter ND = 3.0
NE40B-A 25.0 mm diameter absorptive ND filter ND = 4.0
These filters are mounted in a 1-inch mounting hole in each of the 5 spaces in the wheel. Next in line are two cage plates (Thorlabs; CP02) each with a 1-inch diameter hole to accept optic components. The first component is a 1-inch diameter 500 nm (green) interference filter (Thorlabs; FD1G). This filter can be changed for another which has different band pass characteristics, depending on the sensitivity of the photoreceptors under study. Such filters are available from Thorlabs. The second component is a 1-inch diameter plano convex lens of focal length 5 cm (Thorlabs; LA1951-A). These two components are connected together by intermediate optical tubes (Thorlabs; SM1L10). The second of these two cage plates is connected to a cage cube-mounted non-polarizing beam splitter (Thorlabs; CCM1-BS013) that contains a mirror to direct light vertically to a 4 mm aperture, over which the retina in physiological solution is placed. A variety of miscellaneous mechanical rods and brackets for mounting components to the base plate can also be purchased from Thorlabs. These include a mounting base (Thorlabs; BA2, BA1S) which connects to the base plate, mounting rods (Thorlabs; ER2, ER3) and mounting posts (Thorlabs; TR2, TR1) that connect components together. Normally, a thin opaque plastic plate or cardboard card is placed between the colored filter and the lens to act as a shutter. Because relatively long times of light exposure are normally used, removal and subsequent insertion of the card provides light stimulation of sufficient accuracy.
The photostimulator can be constructed using the following parts (Figure 1):- Tungsten-halogen lamp (Thorlabs, catalog number: QTH10B)
- Aluminum plate (Thorlabs, catalog number: MB1218)
- Filter wheel with positions for neutral density filters (Thorlabs, catalog number: CFW6)
- Neutral density filters (Thorlabs, catalog numbers: NE05B-A; NE10B-A; NE20B-A; NE30B-A; NE40B-A)
- Cage plates (Thorlabs, catalog number: CP02)
- Interference filter (Thorlabs, catalog number: FD1G)
- Plano convex lens (Thorlabs, catalog number: LA1951-A)
- Intermediate optical tube (Thorlabs, catalog number: SM1L10)
- Cage cube-mounted non-polarizing beam splitter (Thorlabs, catalog number: CCM1-BS013)
- Mounting base (Thorlabs, catalog numbers: BA2; BA1S)
- Mounting rods (Thorlabs, catalog numbers: ER2; ER3)
- Mounting posts (Thorlabs, catalog number: TR1)
- A black, light-tight Delrin plastic box (Figure 2)
Used for incubation of the bleached retinal tissue. Incubation is optionally performed for various periods in darkness following light exposure to test the persistence of rhodopsin phosphorylation in darkness. - Fluorescent light (B&H, Porta-trace/Gagne LED light box (11”x18”), catalog number: POLB1118L2)
- Fluorescent light–Lithonia lighting (Aubuchon Hardware, catalog number: 676883)
- Homogenizer (Kinematica, Polytron, model: PT1200C)
Note: Three tips are available for Polytron PT-1200C, each optimized for different volumes. We recommend the tip optimized to homogenize ~0.1-5 ml of sample (Kinematica, model: PT-DA 1205/2EC). The tip speed (max.) used is 3.5 m/s. - Hoefer dual gel caster (Hoefer, catalog number: SE6015)
- Clamp assembly (Hoefer, catalog number: SE6003U)
- Cams (Hoefer, catalog number: SE6005L)
- Spacer (Hoefer, catalog number: SE6119-2-.75)
Note: Refer to the Hoefer catalog for protein electrophoresis http://www.harvardapparatus.com/media/harvard/pdf/Hoefer_Catalog_Protein_Electrophoresis.pdf. - IEF system (GE Healthcare, Amersham Pharmacia, model: Multiphor II system)
- Cooling system (GE Healthcare, Amersham Pharmacia, model: MultiTemp III system)
- Electrofocusing strips (GE Healthcare, Amersham Pharmacia, catalog number: 18100440)
- Sample application strip (GE Healthcare, catalog number: 18-1002-26)
- Power supply (Bio-Rad, model: PowerPac 3000)
- Centrifuge
- 4 °C refrigerator
- -80 °C freezer
- Dim red light bulb (Amazon, Delta 1 Brightlab Universal Red Junior Safelight 11 W bulb, catalog number: 35110)
- Nutator (VWR International, catalog number: 82007-202)
- Curved tweezers (Electron Microscopy Sciences, catalog number: 78752-11)
- Scalpel (Electron Microscopy Sciences, catalog number: 72042-11)
Software
- ImageJ (NIH/https://imagej.nih.gov/ij/)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Lokappa, S. B., Cornwall, M. C. and Chen, J. (2019). Isoelectric Focusing to Quantify Rhodopsin Phosphorylation in Mouse Retina. Bio-protocol 9(14): e3300. DOI: 10.21769/BioProtoc.3300.
- Berry, J., Frederiksen, R., Yao, Y., Nymark, S., Chen, J. and Cornwall, C. (2016). Effect of rhodopsin phosphorylation on dark adaptation in mouse rods. J Neurosci 36(26): 6973-6987.
分类
生物化学 > 蛋白质 > 电泳
神经科学 > 感觉和运动系统 > 视觉系统
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link