- EN - English
- CN - 中文
Dynamic and Sequential Protein Reconstitution on Negatively Curved Membranes by Giant Vesicles Fusion
巨型囊泡融合在负曲面膜上的顺序蛋白重建及动态
发布: 2019年07月05日第9卷第13期 DOI: 10.21769/BioProtoc.3294 浏览次数: 4982
评审: Samantha E. R. DundonAnonymous reviewer(s)

相关实验方案
基于蛋白筛选策略从合成文库中分离抗原特异性纳米抗体:结合 MACS 的酵母展示筛选与 FLI-TRAP 方法
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
2026年01月20日 406 阅读
Abstract
In vitro investigation of the interaction between proteins and positively curved membranes can be performed using a classic nanotube pulling method. However, characterizing protein interaction with negatively curved membranes still represents a formidable challenge. Here, we describe our recently developed approach based on laser-triggered Giant Unilamellar Vesicles (GUVs) fusion. Our protocol allows sequential addition of proteins to a negatively curved membrane, while at the same time controlling the buffer composition, lipid composition and membrane tension. Moreover, this method does not require a step of protein detachment, greatly simplifying the process of protein encapsulation over existing methods.
Keywords: GUV (GUV)Background
Cellular membrane remodeling processes including vesicle trafficking can be topologically split into either outside-in budding such as endocytosis and inside-out budding such as intraluminal vesicle formation at early endosomes, enveloped virus budding and cytokinesis. A budding event induces changes in membrane shape, resulting in two distinct topologies: a convex surface or a concave surface, conventionally defined as positive and negative curvature, respectively (Figure 1).
In vitro methods for studying outside-in budding processes based on membrane nanotube pulling such as the interaction of proteins with positively curved membranes during endocytosis are well-established (Sorre et al., 2012; Prévost et al., 2017). In contrast, investigating protein interaction with negatively curved membranes is much more challenging because it is in general not straightforward to obtain such a topology–i.e., to incorporate proteins inside a membrane nanotube pulled from a GUV in a classic tube-pulling experiment. Indeed, the membrane has to be deformed away from the compartment where proteins are present (Figure 1). Encapsulating the protein of interest inside the GUV can in principle be achieved during GUV swelling, and by pulling out a nanotube afterwards (Prévost et al., 2015). This methodology, however, requires the protein to be detached from the Outer leaflet of the GUV, which can only be performed if the binding is reversible, and the procedure has to be adjusted for each individual protein. Moreover, when a protein is encapsulated, it also interacts with the membrane during swelling, potentially hampering the process of swelling itself. Another limitation of this protocol is that sequential addition of proteins (or lipids) is not possible.
An alternative method consists in pulling a nanotube inside the GUV, and adding the protein from the outside by micropipette injection (Dasgupta and Dimova, 2014). But this method requires very flaccid GUVs, a combination of flow and optical tweezers to form the tube, and is not suitable when the tube diameter has to be controlled down to 15-20 nm.
Here we describe a protocol to investigate the interaction of proteins with negatively curved membranes and membrane necks (De Franceschi et al., 2018). We apply this method to study the endosomal sorting complex required for transport (ESCRT) machinery, which catalyzes inside-out membrane budding processes by polymerizing on negatively curved membranes that carry a negative charge (Henne et al., 2013; McCullough et al., 2018; Caillat et al., 2019). Characterizing the ESCRT-III complex is particularly challenging, because these proteins exhibit strong binding to the membrane, so that their removal from the Outer membrane of a GUV after the encapsulation process is impossible (De Franceschi et al., 2018), making other methods (Prévost et al., 2015) unsuitable. Our method relies on laser-triggered GUV fusion (Rørvig-Lund et al., 2015), and presents a number of advantages over existing methods. First, protein encapsulation is performed in conditions that inhibit protein binding to the membrane, avoiding inhibition of GUV swelling and protein/lipids aggregation. Thus, the protein can be encapsulated using a buffer that keeps it stable and avoids unwanted in-bulk polymerization. However, upon fusion with a protein-free GUV, optimal binding conditions, including lipid species and salt concentration, can be achieved. Importantly, GUV fusion is triggered after a membrane nanotube has been pulled, therefore allowing visualizing dynamic interaction of proteins with a negatively-curved membrane topology. Moreover, by performing multiple fusion events, additional proteins can be added sequentially. Similarly, the lipid composition can be modulated in a controlled fashion by sequential fusion events.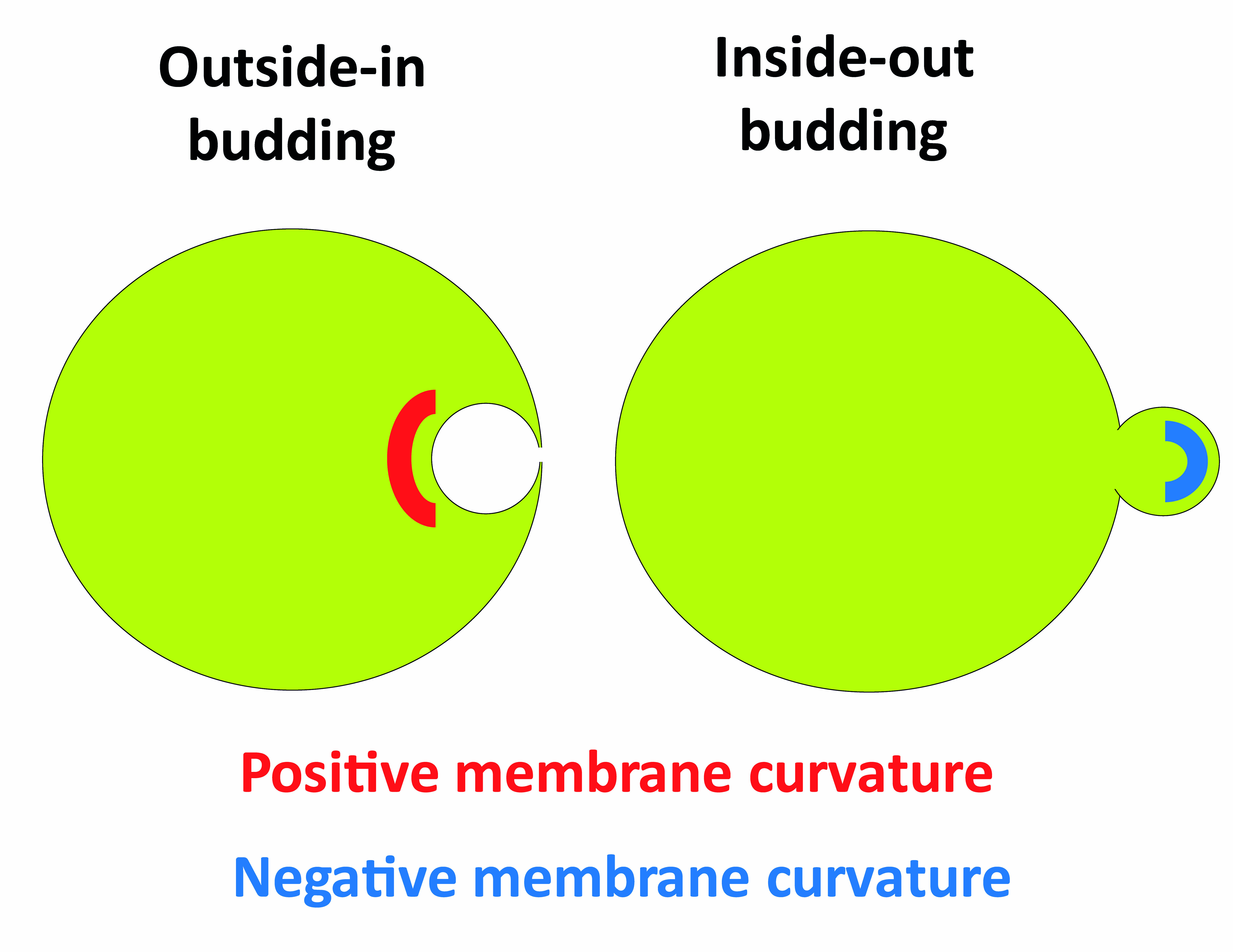
Figure 1. Schematic representing outside-in and inside-out budding events, and the membrane curvatures created during these processes
Method outline: The principle in this methodology is to have proteins inside one neutral GUV (green in Figure 2) and a second protein-free GUV made of negatively charged lipids (magenta in Figure 2) until GUV fusion is triggered. A tube is pulled beforehand from the negatively charged GUV (magenta), so that upon GUV fusion negative membrane curvature becomes accessible to the proteins. Therefore, the lipid mix and buffer in the green GUV are designed to maintain proteins' stability and allow their encapsulation avoiding premature binding and aggregation. At the same time, the final lipid and buffer composition resulting from GUV fusion must create the appropriate conditions for protein-membrane interaction.
We exemplify this concept by explaining the design of buffers and lipid mixes used for investigating binding between the ESCRT-III protein CHMP2B and PI(4,5)P2 (De Franceschi et al., 2018).
CHMP2B is stable at relatively high salt concentration. Lowering NaCl concentration to 50 mM in the presence of negatively charged lipids such as PI(4,5)P2 triggers polymerization and membrane binding. Therefore, CHMP2B is encapsulated in Buffer E containing 70 mM NaCl using Lipid Mix E, which does not contain any negatively charged lipids. In these conditions, CHMP2B does not interact with the membrane. Therefore, the proteins present outside the GUVs can be easily washed away by dilution (Figure 2).
The second population of GUVs contains PI(4,5)P2 in a lipid mix that mimics the composition of the inner leaflet of the plasma membrane (Prévost et al., 2015), Here, the buffer P has a low NaCl concentration, so that, upon GUV fusion, it will mix with buffer E resulting in optimal NaCl concentration to promote CHMP2B:PI(4,5)P2 interaction.
A further constrain in the buffers' composition is that the inner buffers (E and P) must be denser than the Outer buffer, to allow the GUVs to sediment both during centrifugation and in the observation chamber (Mathivet et al., 1996). Moreover, all buffers must have the same osmolarity. By using a combination of sucrose, glucose and NaCl, these conditions can be achieved (see Recipes section).
The proteins present outside the GUVs are removed by sequential dilution, and at the same time streptavidin-coated nanorods that interact with biotinylated lipids, are added (Figure 2). Once the GUVs are properly positioned, irradiation of the nanorods at the GUVs' interface will induce a strong local heating, in turn causing membrane instability and thus fusion (Rørvig-Lund et al., 2015). We improved the efficiency of this method by directly tethering the nanorods to the membrane via streptavidin:biotin interaction.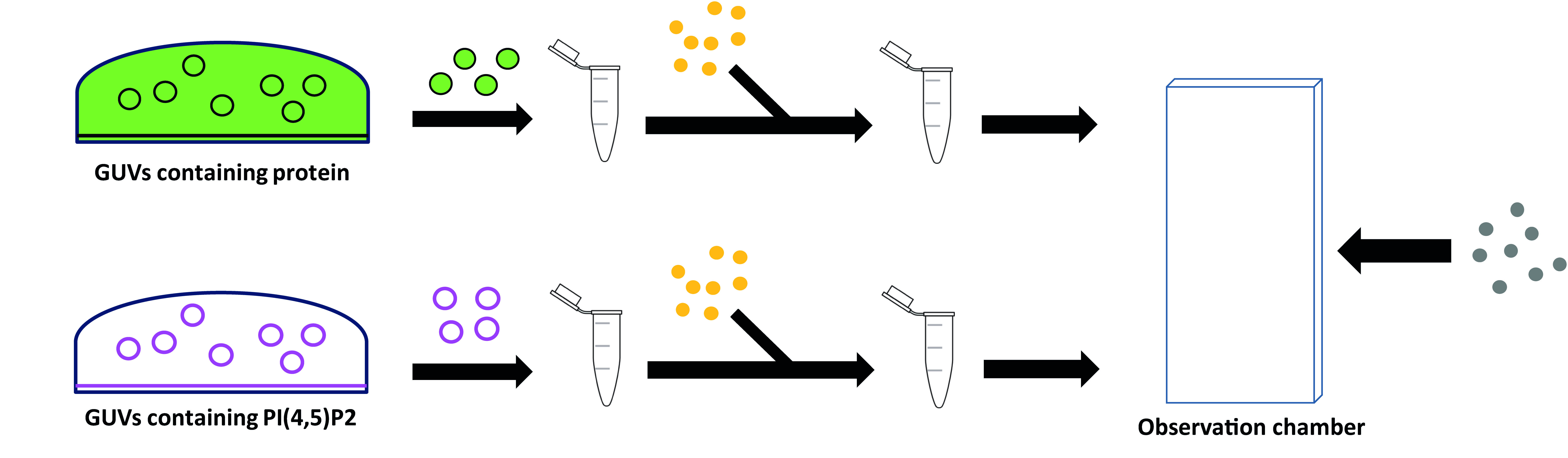
Figure 2. Stepwise procedure for protein encapsulation and washing. Two populations of GUVs are grown separately, then diluted in Outer buffer and centrifuged. GUVs collected from the bottom of the Eppendorf are added to Streptavidin-coated Gold nanorods (in yellow) and further diluted in Outer buffer before being introduced to the observation chamber together with the Streptavidin-coated microbeads (in grey).
Materials and Reagents
- Consumables
- Pipette tips
- 18 x 18 mm glass coverslips (Menzel, catalog number: BB018018A1)
- Hamilton syringe (Dominique Dutscher, Type 701/N)
- 3.5 mm culture dish (Sigma-Aldrich, catalog number: CLS430165)
- 1.5 ml centrifuge tube (Sigma-Aldrich, catalog number: T9661)
- 0.5 ml tube (Sigma-Aldrich, catalog number: T9036)
- 0.22 µm membrane filter (MF-MilliporeTM, catalog number: GSWP04700)
- 1 ml syringe (Sigma-Aldrich, catalog number: Z230723).
- Kimtech precision tissue task wipe (Kimtech, catalog number: 05511)
- Lipids
Note: All lipids were purchased in powder from Avanti Polar Lipids, dissolved in chloroform (unless otherwise indicated) and stored at -20 °C.- Egg L-α-phosphatidylcholine [EPC] (Avanti Polar Lipids, catalog number: 840051)
- 1,2-dioleoylsn-glycero-3-phospho-L-serine [DOPS] (Avanti Polar Lipids, catalog number: 840035)
- 1,2-dioleoyl-sn-glycero-3-phosphatidylethanol-amine [DOPE] (Avanti Polar Lipids, catalog number: 850725)
- Cholest-5-en-3β-ol [cholesterol] (Avanti Polar Lipids, catalog number: 700000)
- L-α-phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] (Avanti Polar Lipids, catalog number: 840046)
Note: Dissolved in chloroform:methanol solution (70:30). - L-α-phosphatidylethanolamine-N- (lissamine Rhodamine B sulfonyl) [egg PE–Rhodamine] (Avanti Polar Lipids, catalog number: 810146)
- 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[biotinyl(polyethyleneglycol)-2000] [DSPE-PEG(2000)-biotin] (Avanti Polar Lipids, catalog number: 880129)
- Other reagents
- β-casein from bovine milk (> 99%) (Sigma-Aldrich, catalog number: C6905)
- Sucrose (Sigma-Aldrich, catalog number: S7903)
- D+ glucose (Sigma-Aldrich, catalog number: G7021)
- NaCl (Sigma-Aldrich, catalog number: S7653)
- Tris (Sigma-Aldrich, catalog number: 10708976001)
- Streptavidin-functionalized gold nanorods (NanopartzTM, catalog number: C12-10-850-TS-DIH-50)
- Polyvinyl alcohol (PVA; Merck, catalog number: 8.14894.0101)
- Polystyrene streptavidin coated beads (diameter 3.2 μm) (Spherotech, catalog number: SVP-30-5)
- Chloroform (Sigma-Aldrich, catalog number: 288306)
- Mineral oil (Sigma-Aldrich, catalog number: M5310)
- Ethanol (96%, Sigma-Aldrich, catalog number: 16368)
- Lipid mix P (see Recipes)
- Lipid mix E (see Recipes)
- Buffer P (see Recipes)
- Buffer E (see Recipes)
- Outer buffer (see Recipes)
- PVA solution (see Recipes)
- β-casein solution (see Recipes)
Equipment
The equipment has been accurately described in Prévost et al. (2017).
- Micropipettes: borosilicate capillaries with internal and external radii of 0.78 mm and 1 mm, respectively (Harvard apparatus, catalog number: 30-0036)
- Phase-contrast microscope: Nikon TE2000 inverted microscope, eC1 confocal system (Nikon), with two laser lines (λ = 488 nm and 543 nm); optical tweezers induced by a 5 W ytterbium fiber continuous wave laser (λ > 1070 nm; IPG GmBH Germany)
- Plasma cleaner (SPI Supplies, Plasma Prep III)
- Oven: Series B Classic.Line (Binder, Mode B 28)
- Vacuum pump: Pfeiffer Balzers Turbo Molecular Pumps DRAG 020 and Pfeiffer Balzers TCP 015 Turbo Molecular Pump Controller
- Glass syringe: Hamilton 701N (10 µl) (VWR, catalog number: 549-1135)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
de Franceschi, N., Alqabandi, M., Weissenhorn, W. and Bassereau, P. (2019). Dynamic and Sequential Protein Reconstitution on Negatively Curved Membranes by Giant Vesicles Fusion. Bio-protocol 9(13): e3294. DOI: 10.21769/BioProtoc.3294.
分类
生物物理学 > 生物工程 > 蛋白封装
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link