- EN - English
- CN - 中文
Oxygen Consumption Measurements in Caenorhabditis elegans Using the Seahorse XF24
利用海马细胞能量代谢实时测定仪XF24 进行秀丽隐杆线虫中耗氧量测定
发布: 2019年07月05日第9卷第13期 DOI: 10.21769/BioProtoc.3288 浏览次数: 6711
评审: Olga SinTugsan TezilAnonymous reviewer(s)
Abstract
Mitochondria generate 90% of the energy required to sustain life. As a result, loss of mitochondrial function compromises almost every facet of human physiology. Accordingly, most mitochondrial diseases tend to present themselves as complex, multi-systemic disorders that can be difficult to diagnose. Depending on the severity of the mitochondrial dysfunction, the pathology can range from mild discomfort to severe epilepsy, blindness and paralysis. To develop therapies to these diseases, it will be important to optimize experimental techniques that can reliably quantify mitochondrial function, particularly in live cells or intact organisms. Here, we describe how a Seahorse XF24 Analyzer can be used to measure both basal and maximal respiration in the nematode Caenorhabditis elegans, and how this data can be interpreted to evaluate mitochondrial function.
Keywords: Oxygen consumption (耗氧量)Background
Energy animates life. Most of the energy that fuels our bodies is generated by mitochondria, small double-membraned organelles that are frequently called the powerhouses of our cells. Along the inner membrane of these organelles, mitochondria house all of the machinery required for energy production. Part of this machinery is encoded by the mitochondrial genome (mtDNA), a small, circular molecule that encodes various subunits of the electron transport chain. Since the discovery of this genome, it has been found that various insults, including exposure to toxins and age-related stress can result in genetic mutations that perturb mitochondrial function (Wallace, 2005). Typically, these genetic anomalies result in decreased energy production, which can drive pathology in a wide variety of tissues and manifest itself at different ages with varying degrees of severity (Wallace, 2005). For example, inherited mtDNA mutations can result in premature deafness, myopathy, or severe encephalomyopathy in children (Saneto and Sedensky, 2013), while age-related mutations contribute to neurodegeneration (Kraytsberg et al., 2006) and muscle wasting (Wanagat et al., 2001) in aging adults. Unfortunately, there are currently no cures for any disease that are caused by mtDNA mutation or depletion.
In order to develop therapies for these diseases, and to better understand the basic biology that underlies mitochondrial function, it will be essential to develop tools that can accurately assess mitochondrial health. Ideally, these tools would be able to measure mitochondrial function in whole cells or animals, so that the effect of pharmacological manipulations can be observed in real time. One of the most successful instruments for the analysis of live cells is the Seahorse XF Analyzer. This instrument was originally designed to record the oxygen consumption rate (OCR) of adherent cells, which serves as a proxy for mitochondrial respiration. In addition, it can record the extracellular acidification rate (ECAR) of the cells, which is a proxy for their glycolytic flux. Both of these parameters can be monitored in real-time in an 8, 24 or 96-well plate, so that multiple cell lines can be tested simultaneously. Moreover, each well of the 8, 24 and 96-well plates is equipped with 4 ports, each of which can hold a separate solution (Figure 1). Each of these solutions can be injected into the wells on demand and subsequently mixed into the solution, so that the cells can be exposed to multiple reagents in succession. This setup is ideal for interrogating individual components of the electron transport chain with oligomycin, FCCP and rotenone. Finally, all of these steps can be pre-programmed, so that the entire protocol can be ran with a single touch of a button.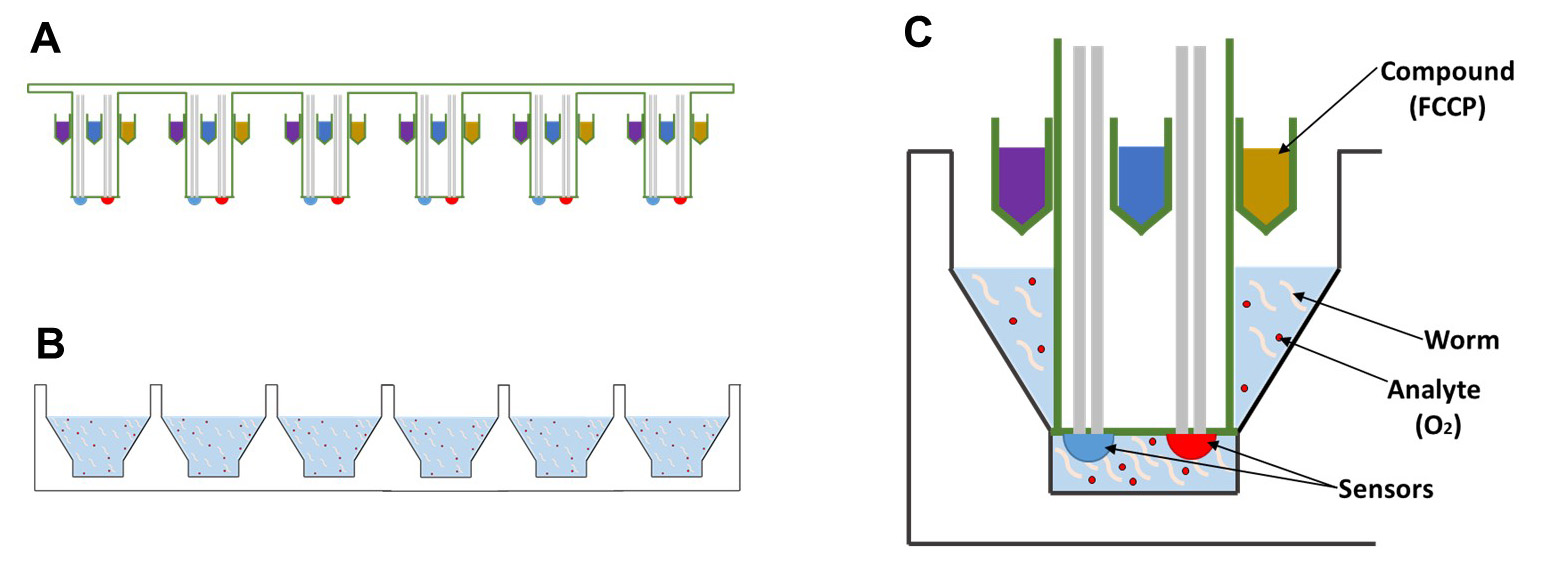
Figure 1. Schematic of the Seahorse XF bio-analyzer plates. Side-view of the (A) sensor cartridge and the (B) cell culture microplate, which will house the animals, are packaged separately. C. Each probe contains 4 injection ports which can be used to add compounds, such as FCCP, and two sensors for oxygen levels and the pH of the solution.
Therefore, we decided to adapt the Seahorse XF bio-analyzer to measure oxygen consumption in C. elegans. Theoretically, other tools might be adapted for this purpose as well, including the Clark electrode; however, these tools tend to be more labor intensive and a better fit for experiments on high-quality mitochondrial extracts. There is one important drawback to the Seahorse XF bio-analyzer as well though. Oxygen consumption measurements with this instrument require a solid-state sensor to be lowered into the wells containing the samples. The proximity of this probe to the samples creates a transient microchamber in which changes in the concentration of oxygen (OCR) and protons (ECAR) can be measured (Figure 1C), which means that these measurements are only accurate if the samples are directly underneath the probe. This requirement creates a problem in the context of worm biology, because the worms are highly mobile, and actively swimming in and out of the microchamber. As a result, it is important for a substantial amount of worms to be present in the well, to ensure that one, or a few worms are always present in the microchamber. To compensate for the remaining variation, multiple replicates must be used of each sample, and numerous measurements must be made for each condition or timepoint. Because the 24-well format of the Seahorse provides a good balance between the number of worms that can be assayed, as well as the number of replicates that can be monitored, we decided to use the Seahorse XF24 instrument for our experiments. Here, we provide a protocol to determine the basic and maximum oxygen consumption rate of mitochondria in intact worms using the XF24 Seahorse Analyzer. This protocol can be used by any researcher with access to the instrument, because it only requires tools to manipulate worms and a microscope equipped with a camera to count them. In doing so, researchers will be able to marry the genetic flexibility of the nematode C. elegans to a powerful screening tool for mitochondrial health and bio-energetic flux. This combination provides a great opportunity to identify novel genetic, biochemical and pharmacological modifiers of mitochondrial function.
Materials and Reagents
- 1,000 μl pipette tips (Neptune, catalog number: 89425-640)
- 200 μl pipette tips (Neptune, catalog number: 89140-900)
- 10 μl pipette tips (Neptune, catalog number: 89140-900)
- 1.5 ml microcentrifuge tubes (VWR, catalog number: 20170-650)
- 60 mm Petri dish (Santa Cruz Biotechnology, catalog number: 351863)
- 25 ml serological pipet (VWR, catalog number: 89130-900)
- 0.22 μm filter (Corning, catalog number: 430767)
- Caenorhabditis elegans (N2, wildtype from CGC)
- Triton X-100 (Fisher Scientific, catalog number: AC327371000)
- NaCl (Fisher Scientific, catalog number: BP358-1)
- Yeast Extract (BD, catalog number: 212750)
- Tryptone (BD, catalog number: 211705)
- Peptone (BD, catalog number: 211677)
- Agar (BD, catalog number: 214530)
- Ethyl Alcohol 200 proof (Greenfield Global, catalog number: 111000200)
- Cholesterol (5 mg/ml in 200 proof ethyl alcohol) (Sigma-Aldrich, catalog number: C8667-1G)
- CaCl2 (1 M, filter sterile) (VWR, catalog number: 97062-590)
- MgSO4 (1 M, filter sterile) (VWR, catalog number: IC191422.5)
- KH2PO4 (1 M, filter sterile) (VWR, catalog number: 71003-456)
- K2HPO4 (1 M, filter sterile) (VWR, catalog number: 470009-788)
- Na2HPO4 (VWR, catalog number: 470302-662)
- Dimethyl Sulfoxide (DMSO) (VWR, catalog number: 97061-250)
- Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (192 μM in DMSO) (Sigma-Aldrich, catalog number: C2920-10MG)
- Potassium phosphate buffer (see Recipes)
- M9 Buffer (see Recipes)
- LB Broth for OP50 bacteria (see Recipes)
- NGM plates (see Recipes)
Equipment
- 1 ml pipetman (Eppendorf, catalog number: 3123000063)
- 200 μl pipetman (Eppendorf, catalog number: 3124000083)
- 20 μl pipetman (Eppendorf, catalog number: 3124000032)
- Seahorse Extracellular Flux Analyzer (XFe24 Analyzer) (Agilent)
- Seahorse XF24 FluxPak (Agilent, catalog number: 100850-001)
Note: It contains 18 XF24 sensor cartridges, 20 XF24 cell culture microplates, and 1 bottle of Seahorse XF Calibrant Solution 500 ml. - 20 °C Incubator (VWR, catalog number: 10753-894)
- Pipette Controller (Brandtech Scientific Inc., catalog number: 26333)
Software
- Seahorse XF24 Analyzer Software (Agilent)
- Microsoft Excel or any other spreadsheet program required for post-experimental analysis.
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Haroon, S. and Vermulst, M. (2019). Oxygen Consumption Measurements in Caenorhabditis elegans Using the Seahorse XF24. Bio-protocol 9(13): e3288. DOI: 10.21769/BioProtoc.3288.
分类
生物化学 > 其它化合物 > 氧
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link