- EN - English
- CN - 中文
Capillary Nano-immunoassay for Quantification of Proteins from CD138-purified Myeloma Cells
毛细管纳米免疫实验定量分析经CD138筛选的骨髓瘤细胞蛋白
发布: 2019年06月20日第9卷第12期 DOI: 10.21769/BioProtoc.3267 浏览次数: 6845
评审: Alessandro DidonnaTanxi CaiPiyali Saha

相关实验方案

SPPL2a抑制剂处理细胞后的CD74N端片段聚集的检测
Rubén Martínez-Barricarte [...] Jean-Laurent Casanova
2019年06月05日 6978 阅读
Abstract
Protein analysis in bone marrow samples from patients with multiple myeloma (MM) has been limited by the low concentration of proteins obtained after CD138+ cell selection. A novel approach based on capillary nano-immunoassay could make it possible to quantify dozens of proteins from each CD138+ purified MM sample in an automated manner. Up to now, the knowledge of protein level in those cells was limited because a relatively small quantity of sample is available after the diagnostic procedure. Moreover, the sample often is required for nucleic acids analysis. We have developed the procedure for obtaining proteins from bone marrow samples preserved in RLT+ buffer, and we have successfully applied this approach for the quantification of proteins in the setting of patients with MM. Proteins are extracted from RLT+ buffer, the content is quantified by total protein assay with WES machine and finally, the particular protein expression level is evaluated using specific antibodies by capillary nano-immunoassay with WES machine. The present protocol enables us to quantify many proteins from a limited amount of sample, without losing the opportunity to obtain nucleic acids at the same time. Proteins are quantified automatically in an assay with a low probability of human errors, which makes it a useful tool for biomarkers development.
Keywords: Protein (蛋白)Background
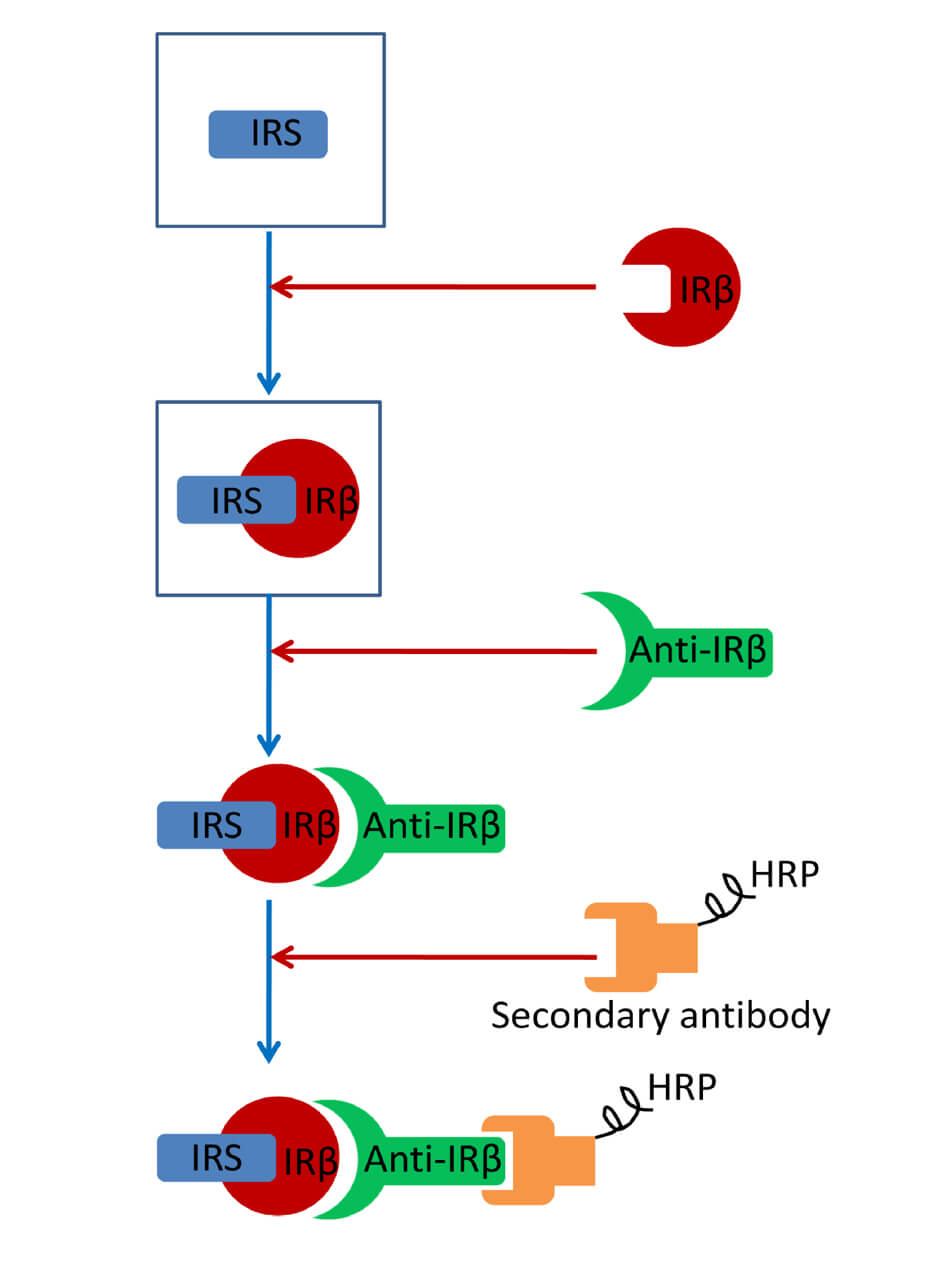
Multiple myeloma (MM) is a clear prototype of a bone marrow-infiltrating tumor for which a relatively small quantity of sample is available after the diagnostic procedure that involves morphological evaluation, immunophenotypic characterization by flow cytometry, and CD138+ plasma cell separation for routine fluorescence in situ hybridization analysis. Protein analysis in bone marrow samples from patients with MM has been limited by the low concentration of proteins obtained after CD138+ cell selection. In the last decades, genomics has dominated biomedical research. In fact, multiple myeloma has been comprehensively analyzed using high-throughput genomic technologies. Particularly, gene expression profiling has provided a molecular classification of MM, which is widely used in biological research. Despite the huge and relevant information obtained from this kind of analysis, it is surprising the very low number of biomarkers subsequently validated. The technological developments that have revolutionized the field of cancer genomics, and in particular of MM, have not progressed to the same extent in proteomics, mostly because the proteome is not as static as the genome, and can differ greatly between individuals and even cell to cell. A novel approach based on capillary nano-immunoassay could make it possible to quantify dozens of proteins from each myeloma sample in an automated manner. We have recently presented a method for the accurate and robust quantification of the expression of multiple proteins extracted from CD138-purified MM samples frozen in RLT+ buffer (QIAGEN), which is commonly used for nucleic acid preservation and isolation (Misiewicz-Krzeminska et al., 2018). Although previous data suggested the possibility of simultaneously extracting proteins and nucleic acids from those kinds of buffer (Chomczynski, 1993; Mathieson and Thomas, 2013; Vorreiter et al., 2016), this strategy has not been applied in the clinical setting. The capillary electrophoresis nano-immunoassay (CNIA) platform has the advantage of measuring protein expression with higher sensitivity and reproducibility compared to traditional Western blot. In addition, the high-throughput screening of samples, using low sample inputs, will allow detecting the relative abundance of protein isoforms in almost all MM patients with the consequent broad applications in MM biomarker discovery. Accordingly, we have demonstrated the biological and clinical value of this analysis for a panel of 12 proteins essential for MM pathogenesis (aiolos, calnexin, cereblon, c-myc, cyclin D1, cyclin D2, DDX21, HSP90, ikaros, PSME1, RIPK1 and XAF1) (Misiewicz-Krzeminska et al., 2018). Moreover, DEPTOR protein level in MM samples was successfully evaluated using this technique (Quwaider et al., 2017). Because of the low sample requirements, low complexity and rapidity of the protocol, these assays can be introduced in almost any laboratory with WES machine in order to evaluate potential protein biomarkers.
Materials and Reagents
- Pipette tips (for instance, 0.1-10 μl, Daslab, catalog number: 162222; 5-200 μl, Daslab, catalog number: 200024; and 100-1,000 μl, Daslab, catalog number: 162001)
- Syringes 1 ml (BD Pastipak, catalog number: 303173)
- Needles 21 G x 1” (Terumo, catalog number: AN*2125R1)
- 1.5 ml and 2 ml Eppendorf tubes (for instance, Sarstedt, catalog numbers: 72.695.500 and 72.690.000)
- JJN3 cell line (DMSZ, catalog number: ACC-541)
- Anti-GAPDH antibody (Cell Signaling, catalog number: 12118), store at -20 °C
- Anti-aiolos antibody (Cell Signaling, catalog number: 12720), store at -20 °C
- DNA/RNA Allprep, including RLT+ buffer (QIAGEN, catalog number: 80204), store at RT
- Acetone (Merck, catalog number: 1000141011), store at RT
- NaCl (Merck, catalog number: 1064041000), store at RT
- Ethanol (Merck, catalog number: 1009832511), store at RT
- NaOH (Merck, catalog number: 1064981000), store at RT
- SDS (Merck, catalog number: 8220501000), store at RT
- Tris-Base (Merck, catalog number: 648310-500GM), store at RT
- Protease inhibitors (Santa Cruz Biotechnology, catalog number: sc29130)
- Anti-Rabbit detection module, includes Luminol-S, Peroxide, Antibody Diluent 2, Anti-Rabbit Secondary Antibody, Streptavidin-HRP (ProteinSimple, Biotechne, catalog number: DM-001) store at 4 °C
- 12-230 kDa Wes Separation Module, 8 x 25 capillary cartridges (ProteinSimple, Biotechne, catalog number: SM-W004), store at RT
- RIPA buffer system (Santa Cruz Biotechnology, catalog number: sc-24948)
- PMSF solution in DMSO, 200 mM (Santa Cruz Biotechnology, catalog number: sc-482875)
- SodProteium orthovanadate in water, 100 mM (New England Biolabs, catalog number: P0758)
- UltraCruz® Protease Inhibitor Cocktail Tablet (Santa Cruz Biotechnology, catalog number: sc-29130)
- Deionized water
- DTT (Promega, catalog number: V3155), store at -20 °C
- Ethanol 70% (see Recipes)
- 5 M NaCl (see Recipes)
- 0.2 M NaOH (see Recipes)
- 1 M Tris, pH 6.8 (see Recipes)
- Protease inhibitors (see Recipes)
- Sample buffer (see Recipes)
Equipment
- Pipettes (volumes needed–10 μl, 2-20 μl, 10-200 μl and 100-1,000 μl)
- WESTM (ProteinSimple, Biotechne, catalog number: 004-600)
- Centrifuge for microtubes (Eppendorf, model: 5415R)
- Freezer -80 °C (Sanyo, model: Vip series 86)
- Freezer -20 °C
- Thermoblock (Grant, catalog number: QB02)
- Vortex (VWR, catalog number: 1444-1372)
- Benchtop centrifuge (VWR, Galaxy MiniStar)
- Centrifuge with the plate adapter (Eppendorf, model: 5810R)
Software
- CompassTM (Protein Simple, San Diego, CA, USA, https://www.proteinsimple.com/software_compass_simplewestern.html)
- Excel (Microsoft)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Misiewicz-Krzeminska, I., Isidro, I. and Gutiérrez, N. (2019). Capillary Nano-immunoassay for Quantification of Proteins from CD138-purified Myeloma Cells. Bio-protocol 9(12): e3267. DOI: 10.21769/BioProtoc.3267.
分类
癌症生物学 > 通用技术 > 免疫学试验
生物化学 > 蛋白质 > 免疫检测 > 免疫印迹法(WB )
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link