- EN - English
- CN - 中文
Separation of Natural Collagen Crosslinks Using Buffer and Ion-pairing Agent Free Solvents on Silica Hydride Column for Mass Spectrometry Detection
二氧化硅氢化物柱配合使用缓冲液和离子配对剂分离获得可通过质谱检测交联的天然胶原蛋白
发布: 2019年05月05日第9卷第9期 DOI: 10.21769/BioProtoc.3224 浏览次数: 4758
评审: Roopali AgarwalVasudevan AchuthanAnonymous reviewer(s)
Abstract
In this protocol we describe the separation of collagen crosslinks in biological tissues and samples including skin, tendon, cartilage, bone and urine. The existing methods use either cation exchange chromatography followed by post-column derivatization with ninhydrin or reverse phase chromatography with mass spectrometry detection. The cation exchange chromatography method has limited sensitivity and long run times while reverse phase chromatography requires strong ion-pairing. In this method, the sample containing crosslinks is applied on a diamond hydride column using water and acetonitrile solvents containing 0.1% (w/v) formic acid. Eight crosslinks are eluted separately from the column and detected by mass spectrometry in the sub-pmol range. By using this method, it is possible to separate all crosslinks of collagen in several biological samples without the need for ion-pairing agent or derivatization for detection.
Keywords: Skin (皮肤)Background
Collagen is the major structural protein and a major component of skin, bone, cartilage, tendon and dentin in teeth. Collagen structure is stabilized by several crosslinks which are found in small amounts in collagen where they are present in different types and varying ratios, depending on the tissue type. Alteration in crosslink types and amount results in several diseases such as bone fragility and skin hyperelasticity (Pinnell et al., 1972 and Arseni et al., 2018).
The development of a sensitive quantitative method using Liquid Chromatography-Mass Spectrometry (LC-MS) is essential for measuring the collagen crosslinks in healthy and diseased tissues. The existing methods for the quantitation of collagen crosslinks including cation exchange chromatography and reverse phase column require sodium buffers and strong ion pairing agents respectively (Avery et al., 2009 and Gineyts et al., 2010). These requirements make them unsuitable for subsequent analysis using Electrospray Ionization Mass Spectrometry (ESI-MS) where high buffer concentrations and ion pairing agents are known to suppress the mass spectrometry signal resulting in significant loss of sensitivity. Also Histidinohydroxylysinonorleucine (HHL) and Histidinohydroxymerodesmosine (HHMD) crosslinks have not previously been detected by mass spectrometry.
We developed a quantitative method for collagen crosslinks on silica hydride column that does not require buffer or ion-pairing agent which allows the use of mass spectrometry (LC-MS) for detection with high sensitivity. Additionally, this method separates and quantitates eight crosslinks in collagenous tissue quicker (total run time less than 10 min) than those of all previously reported methods. This method allows the detection of collagen crosslinks in a complex mixture using MS without the need for buffers or ion-pairing agents.
Materials and Reagents
- Pipette tips
- 1-10 μl (Thermo Fisher, FinntipTM, catalog number: FNP4641040N)
- 10-100 μl (Thermo Fisher, FinntipTM, catalog number: FNP4641070N)
- 100-1,000 μl (Thermo Fisher, FinntipTM, catalog number: FNP4641100N)
- Desmosine (DES) (Sigma-Aldrich, catalog number: D9439)
- Dihydroxylysinonorleucine (DHLNL) (Santa Cruz Biotechnology, catalog number: sc-207059)
- Lysinonorleucine (LNL) (TRC-Canada, catalog number: L488750)
- Pyridinoline (PYR) (BOC Science, catalog number: 77464-35-8)
- Deoxypyridinoline (DPYR) (BOC Science, catalog number: 83462-55-9)
- Hydroxylysinonorleucine (HLNL) (purified in our laboratory, see Naffa et al., 2019a)
- Histidinohydroxylysinonorleucine (HHL) (purified in our laboratory, see Naffa et al., 2019a)
- Histidinohydroxymerodesmosine (HHMD) (purified in our laboratory, see Naffa et al., 2019a)
- Mass spectrometry grade water (Fisher Chemicals, catalog number: FSBW5-4)
- Mass spectrometry grade acetonitrile (Fisher Chemicals, catalog number: FSBA955-4)
- Mass spectrometry grade formic acid (Fisher Chemicals, catalog number: FSBA117-50)
- Acetic acid (UNIVAR, catalog number: UN2789)
- Solvent A (see Recipes)
- Solvent B (see Recipes)
Equipment
- Autosampler amber vials (32 mm x 11.6 mm) (Thermo Fisher, catalog number: THC11090519)
- Vortex (Fisher Scientific, catalog number: FB15012)
- Automated finnpipettes (Thermo Fisher, catalog number: FNP4700850N)
- Thermo Fisher UHPLC with a Q-Exactive Orbitrap Mass Spectrometer equipped with a heated electrospray ionization (HESI) (Thermo Fisher, CA, USA)
Note: Table 1 shows all the specifications of the instrumentation in Equipment 4.
Table 1. Specifications of the Q-Exactive mass spectrometer UHPLC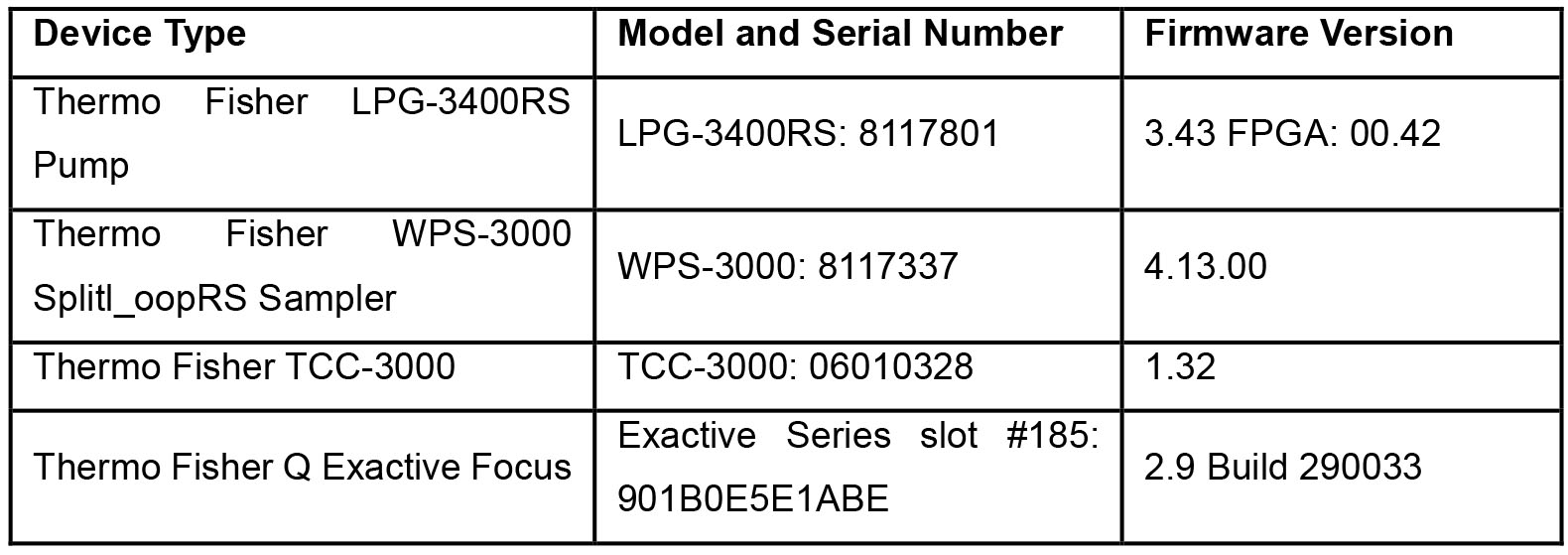
- Cogent Diamond Hydride HPLC column 150 mm x 2.1 mm; particle size, 2.2 µm; pore size 100 Å (Microsolv Technology, catalog number: 70200-15P-2)
Software
- Thermo Xcalibur 4.1.31.9 June 8, 2017 for qualitative analysis and quantitation analysis (Thermo Fisher, CA, USA)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Naffa, R. and Pesek, J. (2019). Separation of Natural Collagen Crosslinks Using Buffer and Ion-pairing Agent Free Solvents on Silica Hydride Column for Mass Spectrometry Detection. Bio-protocol 9(9): e3224. DOI: 10.21769/BioProtoc.3224.
分类
生物化学 > 蛋白质 > 定量
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link