- EN - English
- CN - 中文
Non-radiometric Cell-free Assay to Measure the Effect of Molecular Chaperones on AMP-activated Kinase Activity
非放射性无细胞分析法测定分子伴侣对于AMP激活激酶活性的影响
发布: 2019年04月20日第9卷第8期 DOI: 10.21769/BioProtoc.3218 浏览次数: 5502
评审: Shailesh KumarSalim GasmiAnonymous reviewer(s)
Abstract
AMP-activated kinase (AMPK) is a trimeric protein holoenzyme with kinase activity. AMPK plays an important role in cellular metabolism and is thought to function as a fuel sensor within the cell, exerting kinase activity to activate energy-conserving pathways and simultaneously inhibit energy-consuming pathways. Traditional in vitro methods to measure AMPK activity to test potential agonists or antagonists utilize radiolabeled ATP with a peptide substrate. Although radiolabeling provides a high level of sensitivity, this approach is not ideal for medium to high-throughput screening, dose-response curves, or kinetic analyses. Our protocol utilizes Invitrogen’s Z’-LYTETM Kinase Assay Kit (Ser/Thr 23 Peptide) to measure changes in the enzymatic activity of AMPKɑ2β1γ1 in the presence of a molecular chaperone. The Z’-LYTETM platform is based on Fluorescence Resonance Energy Transfer (FRET). The AMPK peptide substrate (S/T 23 peptide: MRPRKRQGSVRRRV) is a self-contained FRET system, using coumarin as the donor and fluorescein as the acceptor. When the peptide is phosphorylated, it is sensitive to cleavage by a site-specific protease. The cleavage of the phospho-peptide eliminates the FRET pair, and the ratiometric analysis of FRET is used as an indirect measure of AMPK kinase activity. This method does not require the use of radiolabeling or antibodies and is used in a multi-well format, with high reproducibility and throughput capabilities.
Keywords: Kinase (激酶)Background
AMPK plays an important role in cellular metabolism (Ross et al., 2016). Dysfunction in AMPK signaling is associated with a wide range of pathophysiological conditions such as cardiovascular diseases, diabetes, cancer, neurological diseases, and aging (Ronnebaum et al., 2014). Therefore, identifying activators and inhibitors of AMPK activity are important both in the development of therapeutics and to study off-target effects of other small molecules. We previously determined the chaperone effect of the enzyme CHIP on AMPKα1 and AMPKα2 holoenyzmes by measuring the phosphorylation of the SAMS peptide in the presence of radiolabeled ATP (Schisler et al., 2013). While sensitive, laboratories commonly utilize a spotting technique (each condition must be spotted individually) that is time sensitive (reactions can saturate within minutes), making dose response experiments and the simultaneous screening of multiple compounds difficult (Hastie et al., 2006). In a more recent study, we adapted a commercial FRET-based assay to study the effect of CHIP mutations on AMPK activity (Shi et al., 2018).
Materials and Reagents
- 384 multi-well plates (MWP) with the following characteristics: black, low-volume, round bottom, non-binding
- PCR strip tubes (or 96 MWP)
- Pipette tips
- Z’LYTETM Kinase Assay Kit-Ser/Thr 23 Peptide (Thermo Fisher Scientific, catalog number: PV4644)
- 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) (CAS Number: 7365-45-9, bioreagent grade)
- Ethylene glycol-bis(2-aminoethylether)-N,N,N,N-tetraacetic acid (EGTA) (CAS Number: 67-42-5, molecular biology grade)
- Polyoxyethylene (23) lauryl ether (Brij-35) (CAS Number: 9002-92-0)
- Magnesium chloride (MgCl2) (CAS Number: 7786-30-3, bioreagent grade)
- Sodium chloride (NaCl) (CAS Number: 7647-14-5, certified ACS)
- Purified and phosphorylated AMPKα2β1γ1 or other active isoforms (commercially available from multiple vendors, or to produce in-house, see Neumann et al., 2003 and Schisler et al., 2013)
- Molecule/compound/protein of interest (in this example, recombinant CHIP protein, commercially available from multiple vendors, or to produce in-house, see Shi et al., 2018).
- HEPES storage buffer (10x) (see Recipes)
- Kinase buffer A (10x) (see Recipes)
Equipment
- Repeat pipettor (to aliquot 1-20 µl)
- Low-volume multichannel pipettor (repeat pipettor recommended, 1-20 µl)
- Incubator or thermal mixer
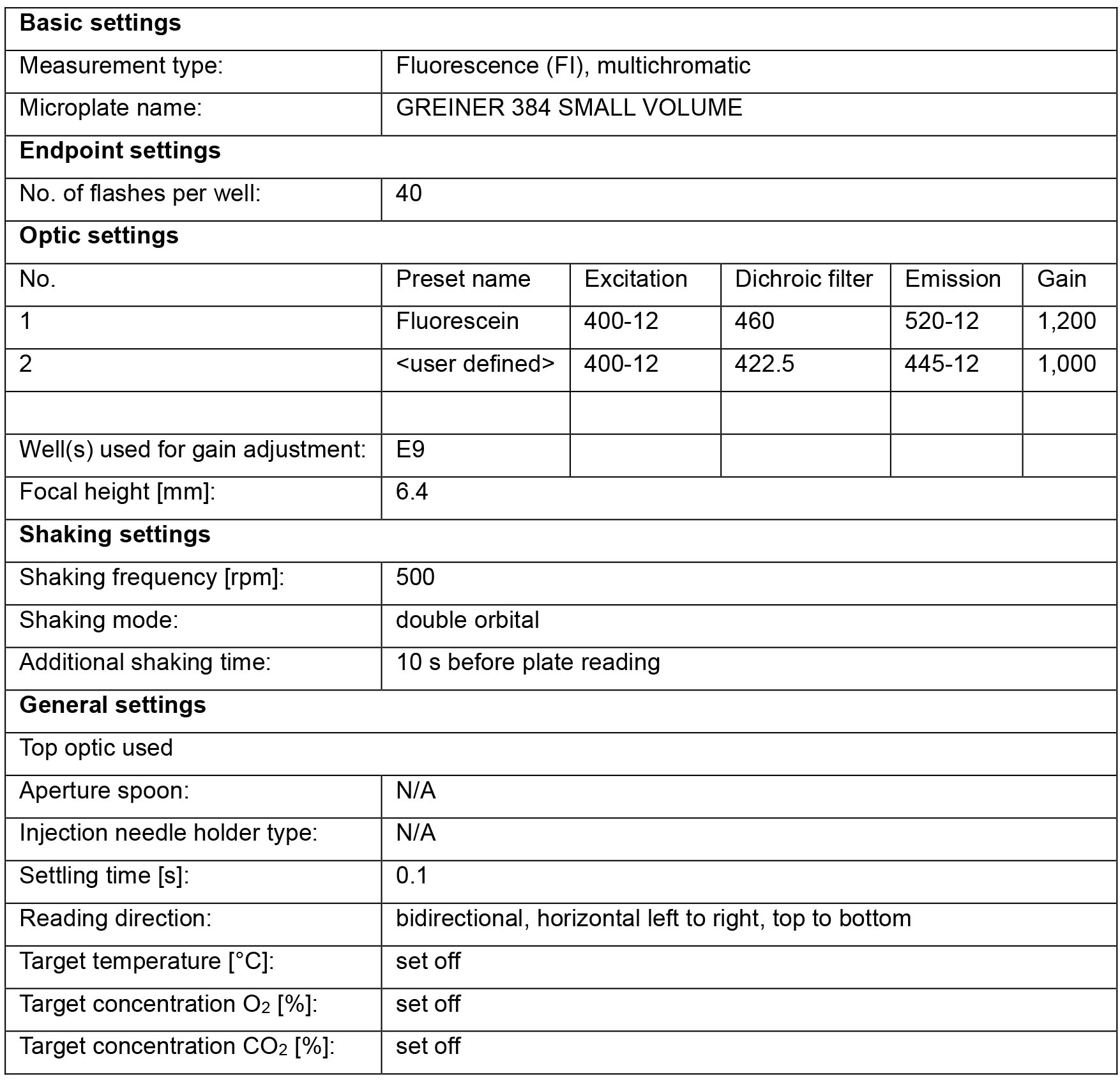
- A fluorescence plate reader with filter sets that detecting the fluorescent emission of coumarin and fluorescein. We use the CLARIOstar (BMG LABTECH) with the following settings:
Software
- MARS Data Analysis Software (v3.31, BMG LABTECH) or equivalent plate reader software.
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Wilson, E. B., Rubel, C. E. and Schisler, J. C. (2019). Non-radiometric Cell-free Assay to Measure the Effect of Molecular Chaperones on AMP-activated Kinase Activity. Bio-protocol 9(8): e3218. DOI: 10.21769/BioProtoc.3218.
分类
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link