- EN - English
- CN - 中文
Differentiation of Human Induced Pluripotent Stem Cells (iPSCs) into an Effective Model of Forebrain Neural Progenitor Cells and Mature Neurons
人诱导多能干细胞 (iPSCs) 分化为前脑神经祖细胞和成熟神经元的有效模型
发布: 2019年03月05日第9卷第5期 DOI: 10.21769/BioProtoc.3188 浏览次数: 17859
评审: Giusy TornilloZe YangAnonymous reviewer(s)

相关实验方案
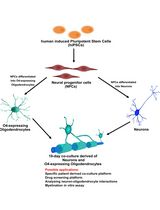
人 iPSC 衍生神经元与少突胶质细胞共培养用于髓鞘形成的小分子筛选分析
Stefanie Elke Chie [...] Maria Consolata Miletta
2025年05月05日 3240 阅读
Abstract
Induced Pluripotent Stem Cells (iPSCs) are pluripotent stem cells that can be generated from somatic cells, and provide a way to model the development of neural tissues in vitro. One particularly interesting application of iPSCs is the development of neurons analogous to those found in the human forebrain. Forebrain neurons play a central role in cognition and sensory processing, and deficits in forebrain neuronal activity contributes to a host of conditions, including epilepsy, Alzheimer’s disease, and schizophrenia. Here, we present our protocol for differentiating iPSCs into forebrain neural progenitor cells (NPCs) and neurons, whereby neural rosettes are generated from stem cells without dissociation and NPCs purified from rosettes based on their adhesion, resulting in a more rapid generation of pure NPC cultures. Neural progenitor cells can be maintained as long-term cultures, or differentiated into forebrain neurons. This protocol provides a simplified and fast methodology of generating forebrain NPCs and neurons, and enables researchers to generate effective in vitro models to study forebrain disease and neurodevelopment. This protocol can also be easily adapted to generate other neural lineages.
Keywords: iPSC (iPSC细胞)Background
Induced pluripotent stem cells (iPSCs) are stem cells produced from non-pluripotent source cells and tissues (Shi et al., 2017). Due to their ability to differentiate into a wide range of cell types, they are a promising avenue for improving our understanding of human development and treatment of degenerative diseases (Marchetto et al., 2011). Of particular interest are iPSC-derived models of human forebrain neurons, as these cells are known to mediate higher order brain functions, including consciousness (Baxter and Chiba, 1999), emotion (Morgane et al., 2005), and sleep (Schwartz and Roth, 2008). As a result, deficits in these cells can cause a wide range of neurological disorders, including neurodegenerative diseases like Alzheimer’s (Auld et al., 2002) and Huntington’s (McColgan and Tabrizi, 2018), as well as neurodevelopmental diseases such as autism (Donovan and Basson, 2017) and epilepsy (Heath, 1976). As there are few effective forebrain models for humans, the discovery of iPSCs spurred a rapid push to develop effective protocols to differentiate iPSCs to forebrain neurons (Srikanth and Young-Pearse, 2014). The first protocols that were developed drew upon previous work using embryonic stem cells (ESCs), which relied upon feeder cell cultures. This complicated the procedure and raised concerns about clinical applications. Later protocols were able to generate forebrain neurons without using feeder cells (Bell et al., 2017), with some eliminating all animal generated products entirely (Yuan et al., 2015). It can be difficult to make an all-encompassing statement about the protocols currently used to generate forebrain NPCs, due to the multitude of labs currently generating forebrain neurons and the many variables that can be changed and optimized. However, many of the recently most cited published protocols for the generation of forebrain NPCs and neurons can be divided into two kinds, monolayer and embryoid bodies (EBs) protocols. In an EB based protocol, iPSCs are dissociated and plated in suspension in a neural induction media to allow them to form EBs, which gradually aggregate over 5-7 days (Pasca et al., 2011). These EBs are then transferred to a plate that supports cell attachment, enabling the embryoid bodies to attach to the bottom of the plate and spread out into a neural rosette. From this rosette, neural stem cells (NSCs) arise, which can be passaged to form relatively stable neural progenitors cells (NPCs) (Shi et al., 2012). NPCs can then be plated in a neuronal induction media to give rise to mature neurons (Bell et al., 2017). Monolayer based protocols chiefly differ in that iPSC colonies are maintained as a monolayer during neural induction, and develop directly into rosettes without aggregation (Chandrasekaran et al., 2017). Using either approach, generation of NPCs from iPSCs is typically reported to require 21-30 days, with electrically active neurons requiring an additional 30+ days of differentiation from NPCs, for a total time of 50+ days to generate forebrain neurons from iPSCs (Yuan et al., 2015).
This protocol describes a methodology for generating forebrain neurons from iPSCs, where iPSC colonies are induced to form neural rosettes without mechanical dissociation, and neural progenitor cells are purified from immature clusters of neural cells, known as neural rosettes based on differential adhesion. Neural progenitor cells will not attach to non-adherent plates and aggregate together in a floating mass, while other cells types either adhere or float but do not aggregate with NPCs (Bell et al., 2017). This allows rapid purification of NPCs, has the potential for automation and enables the generation of NPC cultures within 14 days of initiation of differentiation. This modification does not appear to negatively influence the fate of the cells, as we observe uniform staining for key neural progenitor cells markers (Zhang et al., 2010; Venere et al., 2012; Zhang and Jiao, 2015). Indeed, we have found that we are capable of recording electrical activity from neurons consistently in as little as five days of differentiation from NPCs. This protocol can be used to generate forebrain neurons simply and effectively for use in investigating neurodevelopment, the etiology of diseases that affect the forebrain, and drug testing.
Materials and Reagents
- For Cell Culture
- 6-well plate (SARSTEDT, catalog number: 83.3920)
- 35-mm dish (SARSTEDT, catalog number: 83.3900)
- 60-mm dish (SARSTEDT, catalog number: 83.3901.300)
- 100-mm dish (SARSTEDT, catalog number: 83.3902.300)
- Petri dishes (Fisher Scientific, catalog number: FB0875713)
- Coverslips (Fisher, catalog number: 12-545-80)
- Liquid nitrogen (PRAXAIR, catalog number: 7727-37-9)
- iPSCs, either derived from somatic cells or thawed from a frozen aliquot
- TeSRTM-E8TM Media (Stem Cell Technologies, catalog number: 05990)
- BrainPhysTM Neuronal Medium (Stem Cell Technologies, catalog number: 05790)
- Matrigel® (Corning, catalog number: 354277)
- KnockOutTM DMEM/F-12 (Thermo Fisher, catalog number: 12660012)
- DMSO (Sigma-Aldrich, catalog number: C6164)
- StemPro NSC SFM (Thermo Fisher, catalog number: A1050901)
- SM1 Neuronal Supplement (Stem Cell Technologies, catalog number: 05711)
- N2 Supplement-A (Stem Cell Technologies, catalog number: 07152)
- BSA (Gibco, catalog number: 16140071)
- Non-Essential Amino Acid (NEAA) (Gibco, catalog number: 11140050)
- SB431542 (Stem Cell Technologies, catalog number: SB431542)
- Noggin (Gibco, catalog number: PHC1506)
- Laminin (Sigma-Aldrich, catalog number: L2020)
- Gentle Cell Dissociation Reagent (Stem Cell Technologies, catalog number:07174)
- Epidermal growth factor (EGF) (Sigma, catalog number: E9644)
- Fibroblast growth factor (FGF) (Sigma, catalog number: F0291)
- Brain-Derived Neurotrophic Factor (GenScript, catalog number: Z03208-25)
- Glial-Derived Neurotrophic Factor (GenScript, catalog number: Z02927-50)
- Accutase (Sigma-Aldrich, catalog number: SCR005)
- DPBS without CaCl2 and MgCl2 (Sigma-Aldrich, catalog number: D8537)
- Neural Induction Medium 1 (see Recipes)
- Neural Induction Medium 2 (see Recipes)
- Neural Progenitor Media (see Recipes)
- Neuronal Media (see Recipes)
- Culture Dish Coating with Matrigel® (see Recipes)
- For Immunocytochemistry (ICC)
- Glass coverslips (Fisher, catalog number: 12-545-81)
- Microscope slides (Fisher, catalog number: 12-552-3)
- Pipette tips, 1 ml (SARSTEDT,70.1186)
- Pipette tips, 200 µl (SARSTEDT,70.1186)
- Pipette tips, 20 µl (SARSTEDT,70.1186)
- 15-ml conical tube (SARSTEDT, 62.554.002)
- Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 252549)
- BSA (Sigma-Aldrich, catalog number: A2058)
- Triton X-100
- DAPI (Thermo Fisher, catalog number: 62248)
- Vectashield® (Vector Labs, catalog number: H-1000)
- Nail Polish (Sally Hansen Insta-Dri Fast-Dry Clear Nail Color)
- Antibodies
Antibodies for iPSCs:- TRA-1-60 (Embryonic Stem Cell Marker Panel, Abcam, catalog number: ab109884)
- SSEA (Embryonic Stem Cell Marker Panel, Abcam, catalog number: ab109884)
- Nanog (Embryonic Stem Cell Marker Panel, Abcam, catalog number: ab109884)
- OCT4 (Stemcell Technologies, catalog number: 60093)
- PAX6 (Stemcell Technologies, catalog number: 60094)
- SOX1 (Stemcell Technologies, catalog number: 60095)
- Nanog (Embryonic Stem Cell Marker Panel, Abcam, catalog number: ab109884)
- Nestin (Stemcell Technologies, catalog number: 60091)
- PAX6 (Stemcell Technologies, catalog number: 60094)
- Tuj1 (Abcam, catalog number: ab14545)
- S100B (Abcam, catalog number: ab52642)
- VGLUT1 (Abcam, catalog number: ab77822)
- GABA (Abcam, catalog number: ab86186)
- GFAP (Abcam, catalog number: ab7260)
- ALEXA 488 anti-mouse (Invitrogen, catalog number: A-11008)
- ALEXA 555 anti-rabbit (Invitrogen, catalog number: A-21422)
- Coating glass coverslips with Poly-ornithine and laminin (see Recipes)
- For Electrophysiology
- Borosilicate pipettes with resistances of 3-6 MΩ (World Precision Instruments, catalog number: 1B150-4)
- Cell strainer (40 µm) (Sigma, catalog number: CLS431750)
- Tetrodotoxin (TTX) (Alomone labs, catalog number: T-550)
- BrainPhysTM Without Phenol Red (Stem Cell Technologies, catalog number: 05791)
- HEPES (Sigma-Aldrich, catalog number: H3375)
- KCl (Sigma-Aldrich, catalog number: 793590)
- Potassium Gluconate (Sigma-Aldrich, catalog number: G4500)
- EGTA (Sigma-Aldrich, catalog number: 324626)
- Mg-ATP (Sigma-Aldrich, catalog number: A9187)
- Creatine phosphate (Sigma-Aldrich, catalog number: CRPHO-RO)
- Guanosine triphosphate (Sigma-Aldrich, catalog number: G8877)
- NMDA (Alomone labs, catalog number: N-170)
- Magnesium Chloride hexahydrate (Sigma, catalog number: M2393)
- Internal pipette solution (see Recipes)
Equipment
- Pipettes (Fisher, catalog number: 4680100)
- Pipette puller (Sutter Instrument, model: P-1000)
- Osmometer (Advanced Instruments, model: 3320)
- Bead bath (Lab Armor, model: M706)
- Fluorescent microscope (Olympus, model: 1X73)
- Recording chamber with six-channel valve controller (Warner Instruments)
- Automatic temperature controller (Warner Instruments, model: TC-324C)
- Micromanipulator (Sutter Instrument, model: MP-225)
- Microelectrode amplifier Multiclamp 770B (Molecular Devices)
- Acquisition system Axon digidata 1550A (Molecular Devices)
- Biological Safety Cabinet Class 2 (Nuaire, Model: NU440400)
- Incubator (Thermo Fisher, catalog number: 51030287)
- Centrifuge (Allegra, model: X-12)
Software
- Clampex 10.5 (Molecular Devices, www.moleculardevices.com)
- GraphPad Prism 7 (GraphPad, www.graphpad.com)
- Excel 2016 (Microsoft, https://products.office.com/en-ca/excel)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Bell, S., Hettige, N. C., Silveira, H., Peng, H., Wu, H., Jefri, M., Antonyan, L., Zhang, Y., Zhang, X. and Ernst, C. (2019). Differentiation of Human Induced Pluripotent Stem Cells (iPSCs) into an Effective Model of Forebrain Neural Progenitor Cells and Mature Neurons. Bio-protocol 9(5): e3188. DOI: 10.21769/BioProtoc.3188.
分类
干细胞 > 多能干细胞 > 细胞分化
神经科学 > 细胞机理 > 细胞分离和培养
细胞生物学 > 细胞分离和培养 > 细胞分化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link