- EN - English
- CN - 中文
A Radioisotope-free Oligosaccharyltransferase Assay Method
一种非放射性同位素寡糖基转移酶测定方法
发布: 2019年03月05日第9卷第5期 DOI: 10.21769/BioProtoc.3186 浏览次数: 6333
评审: Alessandro DidonnaAswad KhadilkarRoger Salvacion Tan
Abstract
Glycosylation of asparagine residues is widespread in Eukarya, and occurs in virtually all Archaea and some eubacterial species. A membrane-bound enzyme, oligosaccharyltransferase, catalyzes the transfer of an oligosaccharide chain from a sugar donor (lipid-linked oligosaccharide, LLO) to an asparagine residue in the consensus sequence, Asn-X-Ser/Thr (X ≠ Pro), in proteins. The in vitro oligosaccharyl transfer assay reaction mixture contains a detergent-solubilized oligosaccharyltransferase (OST), a sugar donor LLO, and a sugar acceptor peptide. Previous assay methods are problematic, in terms of the use of radioactive compounds and the cumbersome separation procedures using lectin binding or two-phase partitioning. Here, we describe a new oligosaccharyl transfer assay method, which is radioisotope-free and relies on a different separation mechanism. The glycopeptide products are separated from unreacted peptides by SDS-PAGE. A fluorescent dye is attached to the peptide substrate during custom peptide synthesis. The fluorescent imaging of the SDS-PAGE gels ensures high sensitivity and quantitative performance. The user-friendly PAGE format is particularly suitable for presentation in scientific papers. For illustrative applications, time-course and peptide library experiments are shown.
Keywords: Oligosaccharyltransferase (寡糖)Background
Glycosylation of asparagine residues, which is also referred to as N-glycosylation, is one of the most ubiquitous protein modifications (Cherepanova et al., 2016). N-glycosylation occurs not only in Eukarya, but also in most Archaea (Larkin and Imperiali, 2011). N-glycosylation is also found in some species in Eubacteria. In the entire complex N-glycosylation process, the transfer of an oligosaccharide chain from a sugar donor to asparagine residues in proteins is the defining event (Figure 1). This reaction is catalyzed by a membrane-bound enzyme, oligosaccharyltransferase (OST or OTase). The acceptor asparagine residues, in principle, reside in the consensus sequence Asn-X-Thr or Asn-X-Ser (or Asn-X-Cys in rare cases), where X can be any amino acid residue except Pro. Eubacterial OST uses an extended version of the 5-residue sequon, Asp-X-Asn-X-Ser/Thr or Glu-X-Asn-X-Ser/Thr (X ≠ Pro). Asp is preferable to Glu at the -2 position, even though the acidic residue at the -2 position is not absolutely required in some eubacterial species (Schwarz et al., 2011). The sugar donor is a type of glycolipid, referred to as a lipid-linked oligosaccharide (LLO). The chemical structure of LLO is an oligosaccharide chain attached to a lipid-phospho carrier. The lipid part is dolichol in Eukarya and Archaea, and undecaprenol in Eubacteria. The phosphate group is basically diphosphate, but some archaeal species belonging to Euryarchaeota (an ancient phylum of Archaea) use monophosphate (Taguchi et al., 2016).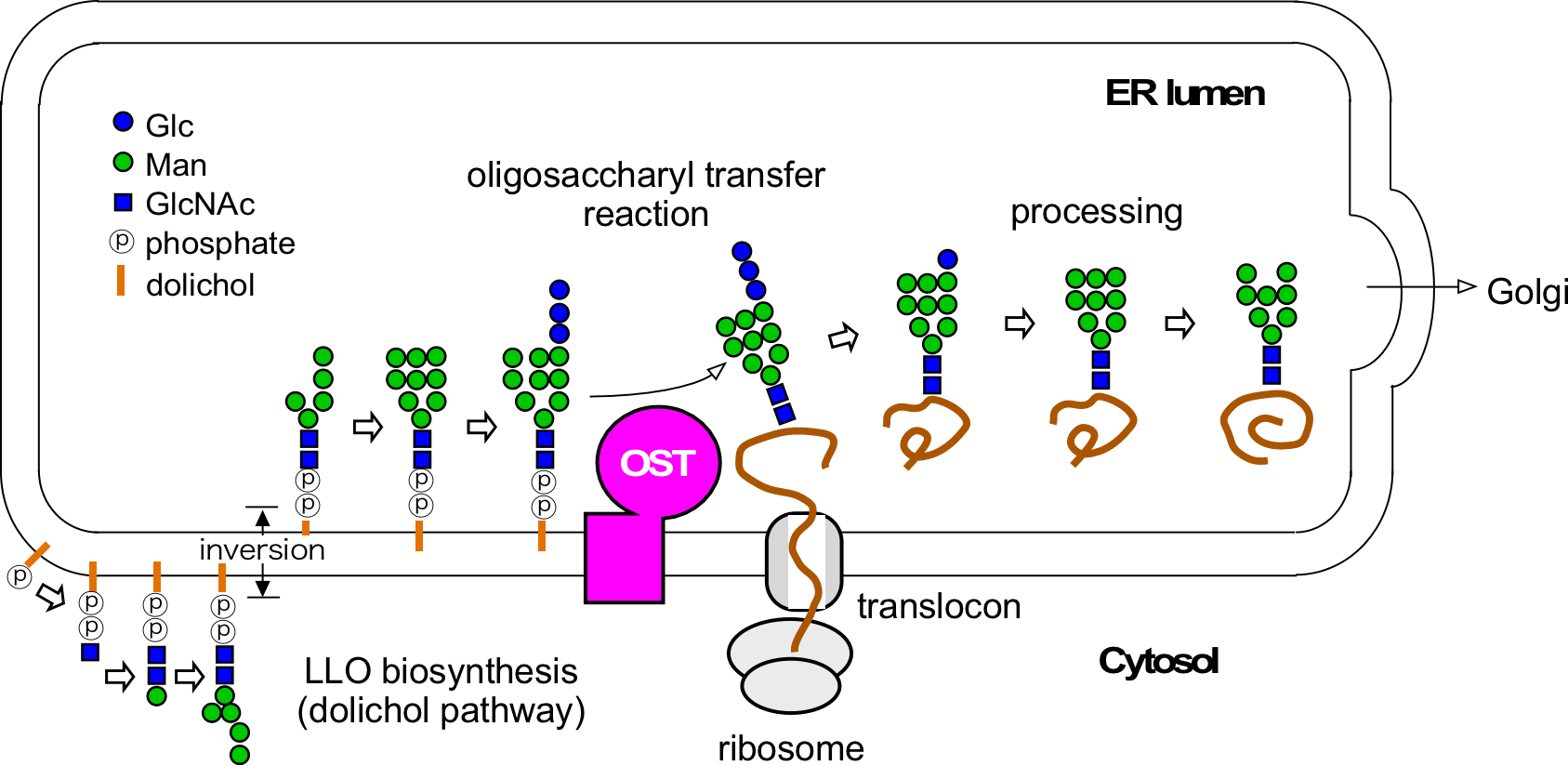
Figure 1. Overview of the biosynthesis of N-glycans attached to proteins. The oligosaccharyltransferase catalyzes the transfer of the oligosaccharide chain from LLO to asparagine residues in nascent proteins on the luminal side of the eukaryotic ER. OST: oligosaccharyltransferase. LLO: lipid-linked oligosaccharide.
The in vivo oligosaccharyl transfer activity is measurable by analyzing the extent of oligosaccharide modification of a specified protein in cells. The amino-acid variants of model glycoproteins can be used to analyze the efficiency of the oligosaccharyl transfer reaction around the N-glycosylation site. However, it is difficult to analyze the effects of amino-acid variation on native oligosaccharyl transfer activity in cells, because the N-glycosylation is essential in eukaryotic cells and rather vital in prokaryotic cells. In general, in-cell studies are difficult to set up. In an exceptional case, the whole N-glycosylation system (the LLO biosynthetic pathway, the OST enzyme, and the substrate protein) of the eubacterium Campylobacter was transferred to Escherichia coli cells (Wacker et al., 2002).
The reaction solution for in vitro oligosaccharyl transfer assay is a mixture of a detergent-solubilized OST enzyme, a sugar donor LLO, and a sugar acceptor peptide. Chromatographically purified LLO is preferred, but crude fractions after two-phase partitioning with a chloroform/methanol/water system are usable. In principle, OST strictly recognizes the chemical structure of LLO. As for eukaryotic OSTs, the chemical structure of LLO (Glc3Man9GlcNAc2-PP-dolichol) is common, and thus the human OST enzyme can utilize LLO isolated from yeast cells. In contrast, the archaeal LLOs are quite structurally diverse and must be isolated from the same or closely related species for the oligosaccharyl transfer assay.
One previous assay method is based on the lectin binding of radioactive glycopeptide products after the oligosaccharyl transfer reaction with a 125I-labeled peptide substrate (Geetha-Habib et al., 1990). Another assay method is based on the recovery of radioactive glycopeptide products in the upper water phase, in two-phase partitioning after the oligosaccharyl transfer reaction with 14C-labeled LLO (Bause and Hettkamp, 1979; Sharma et al., 1981). The two methods are problematic, in terms of the use of radioactive compounds and the cumbersome separation procedures.
Therefore, we have developed a new oligosaccharyl transfer assay method, which is radioisotope-free and highly efficient, by using PAGE as the separation mechanism (Figure 2) (Kohda et al., 2007). The bulky oligosaccharide chain in glycopeptides decreases the rate of migration during electrophoresis, due to the sieving action of the polyacrylamide gel. The glycopeptide products are detected and quantified by fluorescent imaging of the PAGE (polyacrylamide gel electrophoresis) gels. A fluorescent dye for detection is attached to a peptide during peptide synthesis. The minimal detectable quantity of the glycopeptide is 1 fmol in routine assay conditions. The excellent linearity over three orders of magnitude for fluorescently labeled peptide amounts up to 1,000 fmol enabled us to quantify the glycopeptide product accurately, for the determination of the Km and Vmax values for peptide substrates (Igura and Kohda, 2011a). At 12 years after its development, the radioisotope-free oligosaccharyltransferase assay method is now widely used in many laboratories.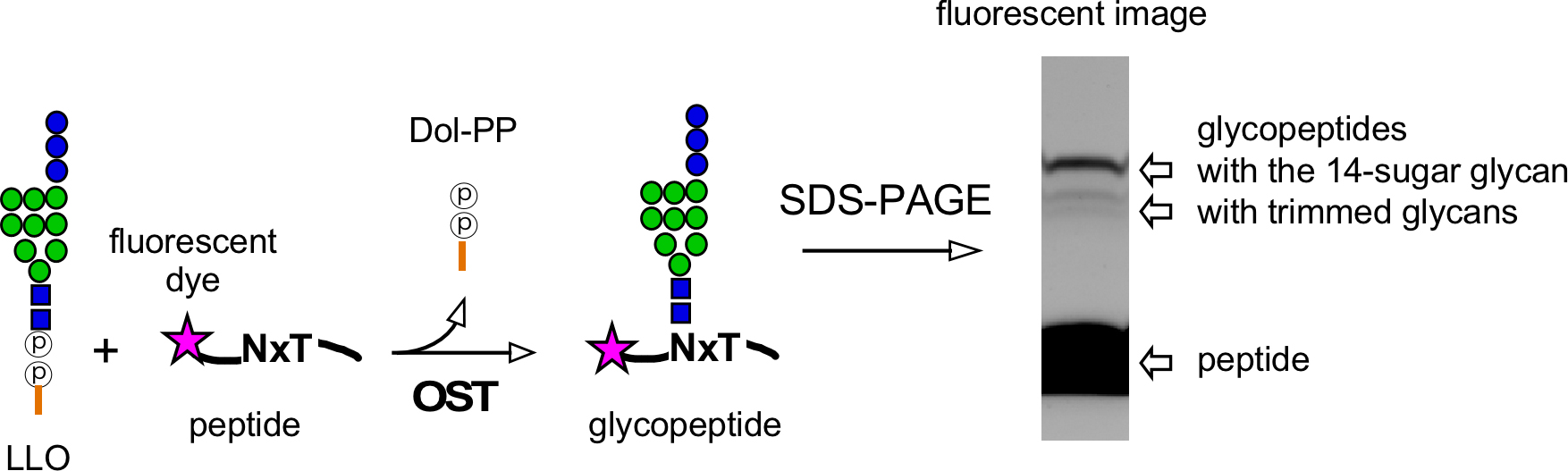
Figure 2. Radioisotope-free assay for the oligosaccharyltransferase activity. Oligosaccharyl transfer assay method using a fluorescently labeled peptide for detection and a separation mechanism based on PAGE (polyacrylamide gel electrophoresis). OST: oligosaccharyltransferase. LLO: lipid-linked oligosaccharide. Dol-PP: dolichol-pyrophosphate
Materials and Reagents
- Labwares
- Pipette tips, 10 μl and 200 μl (Gilson, Diamond tips D10 and D200, catalog numbers: F161630 and F161930)
- Conical polypropylene centrifuge tubes, 15 ml and 50 ml (Thermo Fisher, Nunc, catalog numbers: 339650 and 339652)
- 1.5 ml polypropylene tubes (Eppendorf, 3810X, catalog number: 0030125150)
- Gloves (Ansell, Microflex, catalog number: NPG-INT)
- OST protein and two substrates
- Oligosaccharyltransferase enzyme, isolated from natural sources or recombinantly expressed, purified in the presence of detergent (see Notes for preparation)
- Sugar donor LLO, (partially) purified from cultured cells (see Notes for preparation)
- Sugar acceptor peptide containing the N-glycosylation sequon in the amino acid sequence and a fluorescent dye attached to the N- or C-terminus for detection (see Notes for design and preparation)
- Reagents, solutions, and buffers
- Precast PAGE gels, 15%-25% gradient gel (size 85 x 90 x 0.9 mm3) (Cosmo Bio, MULITGEL II Mini 15/25 (17W), catalog number: DCB-414923)
- Trizma base [tris(hydroxymethyl)aminomethane] (Sigma-Aldrich, catalog number: T1503)
- HCl (Sigma-Aldrich, catalog number: H1758)
- MgCl2 hexahydrate (Sigma-Aldrich, catalog number: M2670)
- MnCl2 tetrahydrate (Sigma-Aldrich, catalog number: 221279)
- Dithiothreitol (DTT) (Sigma-Aldrich, catalog number: 43819)
- Tween 20 (Sigma-Aldrich, catalog number: P1379)
- Lyophilized peptide powder (custom order, TORAY)
- Triton X-100 (Sigma-Aldrich, catalog number: X100)
- N-dodecyl-β-D-maltoside (DDM) (DOJINDO, catalog number: D316)
- Chloroform (Sigma-Aldrich, catalog number: C2432)
- Methanol (Sigma-Aldrich, catalog number: 34860)
- SDS (Sigma-Aldrich, catalog number: L3771)
- Glycerol (Sigma-Aldrich, catalog number: G5516)
- Bromophenol blue (Sigma-Aldrich, catalog number: B0126)
- Glycine (Sigma-Aldrich, catalog number: G8898)
- (Optional) EDTA (ethylenediaminetetraacetic acid) (Sigma-Aldrich, catalog number: EDS)
- 10:10:3 (v/v/v) chloroform:methanol:water (see Recipes)
- 5x SDS-PAGE sample loading buffer (see Recipes)
- Running electrophoresis buffer (see Recipes or Sigma-Aldrich, 10x concentrate, catalog number: T7777-1L)
Equipment
- Micropipettes (Gilson, Pipetman P2, P20, P200, catalog numbers: F144801, F123600, F123601)
- Electronic pipettes (Gilson, Pipetman P10M, P100M, catalog numbers: F81022, F81013)
- 4 °C refrigerator (PHC, MPR-1014)
- Refrigerated laboratory tabletop centrifuge (Eppendorf, Centrifuge 5418R, catalog number: 5401000030)
- Water-bath type sonicator (Branson, Bransonic 2800 ultrasonic cleaner)
- Aluminum heating/cooling block for 1.5 ml tubes (Sigma-Aldrich, catalog number: Z740272-1EA)
- Incubator, natural convection oven or forced air flow convection oven (YAMATO, IC402, catalog number: 211195 or EYELA, NDO-420 or WFO-420, catalog numbers: 252240 or 252280)
Note: Alternatively, a water bath-type incubator and PCR equipment can be used. - Vacuum concentrator that withstands organic solvent (Thermo Fisher, Savant SpeedVac SPD120P1, catalog number: SPD120P1-115/230)
- Cassette electrophoresis unit for PAGE (Cosmo Bio, Model DPE-1020 for Mini Gel, DCB-303111)
- Power supply for PAGE (Bio-Rad, PowerPac Basic, catalog number: 1645050)
- LAS-3000 multicolor gel image analyzer (Fuji Film)
- LED (Green 520 nm EPI) illuminator (for TAMRA dye) and LED (Blue 460 nm EPI) illuminator (for 5-CF/5-FAM dye)
- 575DF20 filter (for TAMRA) and Y515-Di filter (for 5-CF/5-FAM)
- Iris 0.85
- Air (gas) duster (Orientech, catalog number: AD400FL)
- Fume hood (Yamato Scientific, catalog number: RFS-120SZ)
- -20 °C freezer (PHC, catalog number: MDF-MU500H-PJ)
Software
- ImageGauge software (Fuji Film)
Note: Alternatively, any software installed in other gel imagers can be used.
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Yamasaki, T. and Kohda, D. (2019). A Radioisotope-free Oligosaccharyltransferase Assay Method. Bio-protocol 9(5): e3186. DOI: 10.21769/BioProtoc.3186.
分类
生物化学 > 糖类 > 寡糖
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












![N-连接寡糖的[2-<sup>3</sup>H] 甘露糖标记和分析](https://en-cdn.bio-protocol.org/imageup/arcimg/20170718074630401.jpg?t=1770228808)