- EN - English
- CN - 中文
Detection of Disulfides in Protein Extracts of Arabidopsis thaliana Using Monobromobimane (mBB)
单溴二甲双胍 (mBB) 检测拟南芥蛋白质提取物中的二硫化物
发布: 2019年03月05日第9卷第5期 DOI: 10.21769/BioProtoc.3183 浏览次数: 6397
评审: Dennis J NürnbergCiaran McFarlaneAgnieszka Zienkiewicz
Abstract
Thiol-disulfide exchange is a key posttranslational modification, determining the folding process of intra- and inter-protein structures. Thiols can be detected by colorimetric reagents, which are stoichiometrically reduced by free thiols, and by fluorescent adducts, showing fluorescence only after thioester formation. We adapted a simple three-step method for detection of disulfide bonds in proteins. After irreversible blocking of protein thiols, disulfide bonds are reduced, followed by the detection of thiols. The approach presented here provides an economical procedure that can be used to obtain a global overview of the thiol-disulfide status of proteins in plants. This method allows the detection of modifications in samples on a gel and can be used for semi-quantitative analysis.
Keywords: Protein labeling (蛋白质标记)Background
The redox status of proteins plays a key role in a number of cellular processes. Thiol-disulfide exchange reactions support the redox control of plant responses to fluctuating light under natural environments via a photochemical electron transfer cascade for posttranslational modification of proteins. Whereas thiols (R-SH) have relatively higher reactivity than other cellular components and can be directly detected by a variety of reagents and separation techniques (Fahey et al., 1980 and 1981; Newton et al., 1981; Riddles et al., 1983; Fenton and Fahey, 1986; Tyagarajan et al., 2003), disulfide bonds (R-S-S-R) have no such chemical characteristics. Therefore, the most common methodology for the detection of disulfide bonds consists of a three-step procedure: blocking of thiols, reduction of disulfide bonds, and subsequent detection of additionally exposed thiols. Dithiothreitol (DTT) is a well-known and frequently used reducing agent for the protection and exposure of thiol groups of proteins. However, the use of DTT in the laboratory is undesirable because of its harmful odor. Moreover, it can subsequently interfere with thiol detection reagents unless it is appropriately removed (Getz et al., 1999). Also, the reducing power of DTT is limited to pH > 7. Tris(2-carboxyethyl) phosphine-hydrochloride (TCEP) can be a promising alternative with advantages of being odorless and more effective in the reduction of disulfide bonds over a wide pH range from 1.5 to 8.5. Monobromobimane (mBB) has been used for fluorescent labeling of low molecular weight thiols by detecting fluorescent derivatives under a fluorescent microscope or with high-performance liquid chromatography (Fahey and Newton, 1987; Meyer et al., 2001). This protocol describes a simple method for discriminate labeling with mBB of free thiols, disulfide bonds, and total thiols in plant protein extracts. Here, we show an example of the procedure using Arabidopsis leaves. Free thiols are labeled without any preprocessing (Figure 1A), whereas labeling of disulfide bonds requires blocking of free thiols by iodoacetamide (IAA) and reduction of disulfide bonds by TCEP before the mBB treatment (Figure 1B). Total labeling of thiols just after TCEP treatment is helpful to estimate the redox state of total thiols in protein extracts (Figure 1C). This simple method allows the detection of modifications in samples on a gel and can be used for semi-quantitative analysis and provides an overview of the redox state of thiol-containing proteins without any special equipment other than conventional protein electrophoresis and imaging systems.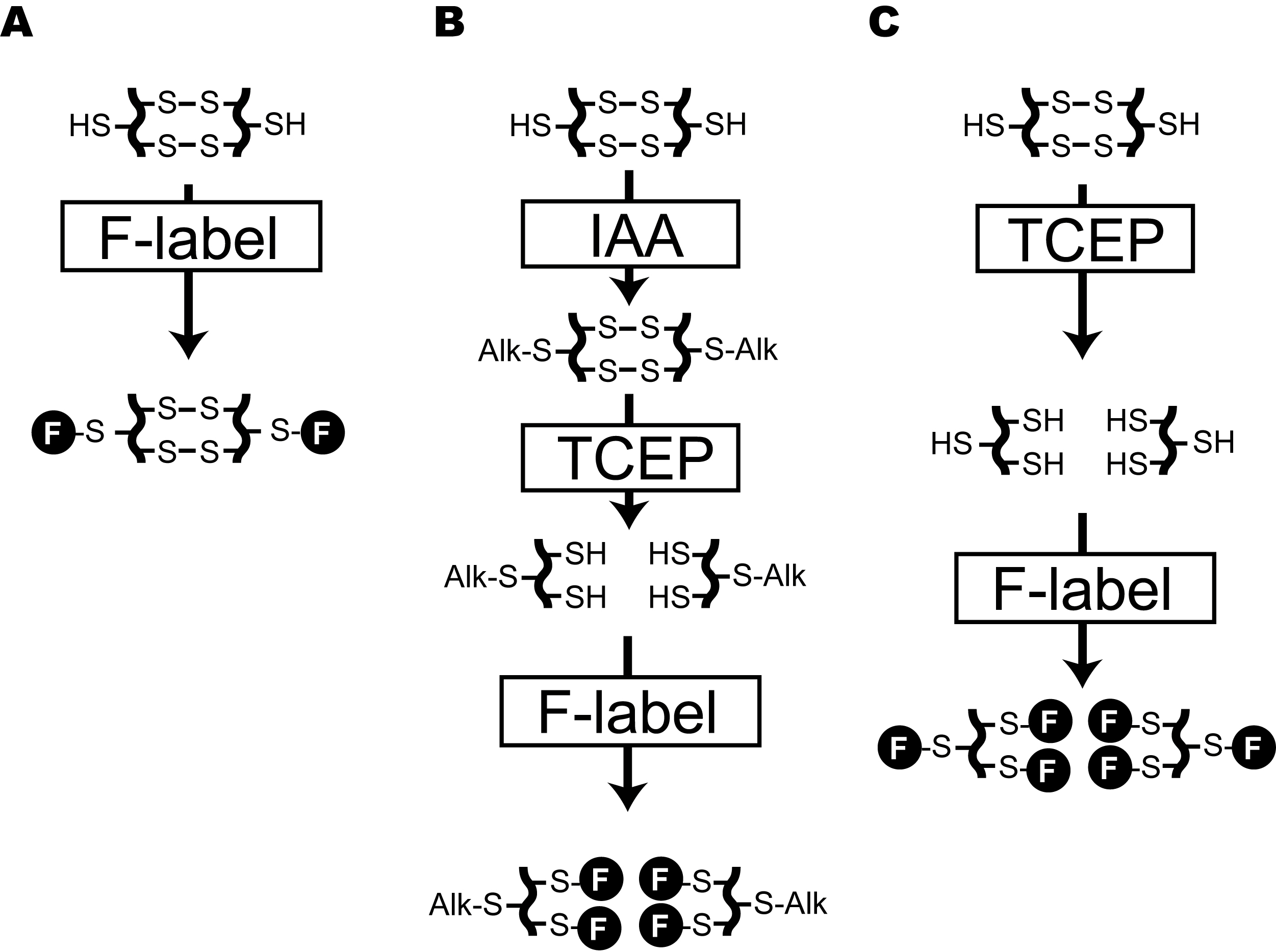
Figure 1. Flowchart for discriminate labeling of thiols in protein under different redox states. A. Labeling of free thiols in proteins. B. Specific labeling of thiols, forming disulfide bonds in protein. C. Total labeling of thiols in proteins. F in closed circle means mBB-derived fluorescence. IAA: Iodoacetamide, TCEP: Tris(2-carboxyethyl)phosphine Hydrochloride.
Materials and Reagents
- Finntip Pipette Tips (Finntip Flex 10, 200, and 1,000)
- 1.5 ml microcentrifuge tube (VIOLAMO, F-1.5, catalog number: 1-1600-01)
- Arabidopsis thaliana wild-type plants (Ecotype: Col-0)
- Arabidopsis thaliana mutant plant of interest (Ecotype: Col-0)
- Liquid nitrogen
- Tris(hydroxymethyl)aminomethane (Tris) (Nacalai Tesque, catalog number: GR35406-91)
- cOmpleteTM, Mini, Protease Inhibitor Cocktail (Roche, catalog number: 4693159001)
- Bio-Rad Protein Assay Kit (Bio-Rad, catalog number: 5000001ja)
- FOCUSTM Protein Alkylation (TaKaRa, catalog number: GA503)
- Iodoacetamide (IAA) (Sigma-Aldrich, catalog number: I1149-5G)
- Trichloroacetic acid solution (TCA), 100% (w/v) (Nacalai Tesque, catalog number: SP06275-24)
- Acetone (Nacalai Tesque, catalog number: SP09888-85)
- FOCUSTM Protein Reductant (TaKaRa, catalog number: GA501)
- Tris(2-carboxyethyl)phosphine Hydrochloride (TCEP) (Nacalai Tesque, catalog number: SP06342-21)
- Monobromobimane (mBB) (Tokyo Chemical Industry, catalog number: B4220)
- Glycerol (Nacalai Tesque, catalog number: SP17045-94)
- Bromophenol Blue (BPB) (Nacalai Tesque, catalog number: B-2609)
- Glycine (Nacalai Tesque, catalog number: SP17141-24)
- Sodium lauryl sulfate (SDS) (Nacalai Tesque, catalog number: SP08933-34)
- Protein extraction buffer (see Recipes)
- IAA solution (see Recipes)
- SDS-PAGE sample buffer (see Recipes)
- SDS-PAGE buffer (see Recipes)
Equipment
- Pipettes (Thermo Fisher Scientific, FinnpipetteTM F2)
- Cork borer (4.0 mm, inside diameter of the hole)
- Conventional Dewar’s vessel
- Vortexer (Scientific Industries, Vortex-Genie 2)
- Homogenizer pestle (VIOLAMO, F-1.5, catalog number:1-2955-02)
- Refrigerated microcentrifuge (TOMY, MX305)
- Absorption spectrometer (Thermo Fisher Scientific, MultiskanTM GO)
- Mini-PROTEAN Tetra System (Bio-Rad, catalog number: 1658004ja)
- Mini-PROTEAN TGX Gels (Bio-Rad, catalog number: 456-1095)
- Power supply (ATTO, catalog number: AE-8135)
- UV Transilluminator (Maestrogen, model: UV-01)
- Yellow filter (Kenko, Black & White Filter, Y2 Professional, λcut-on 470-480 nm)
- Conventional digital camera with hood (RICHO, WG-4)
Software
- ImageJ (https://imagej.nih.gov/ij//)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Hashida, S. and Kawai-Yamada, M. (2019). Detection of Disulfides in Protein Extracts of Arabidopsis thaliana Using Monobromobimane (mBB). Bio-protocol 9(5): e3183. DOI: 10.21769/BioProtoc.3183.
分类
植物科学 > 植物生物化学 > 蛋白质 > 修饰
生物化学 > 蛋白质 > 修饰
生物化学 > 其它化合物 > 硫醇
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link