- EN - English
- CN - 中文
Click Chemistry (CuAAC) and Detection of Tagged de novo Synthesized Proteins in Drosophila
果蝇中通过“链接”化学检测标记的从头合成蛋白
发布: 2019年01月20日第9卷第2期 DOI: 10.21769/BioProtoc.3142 浏览次数: 11660
评审: Martin V KolevTzvetina BrumbarovaAnonymous reviewer(s)

相关实验方案
Cluster FLISA——用于比较不同细胞系蛋白表达效率及蛋白亚基聚集状态的方法
Sabrina Brockmöller and Lara Maria Molitor
2025年11月05日 1205 阅读
Abstract
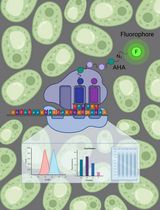
Copper-catalyzed azide-alkyne-cycloaddition (CuAAC), also known as ‘click chemistry’ serves as a technique for bio-orthogonal, that is, bio-compatible labeling of macromolecules including proteins or lipids. Click chemistry has been widely used to covalently, selectively, and efficiently attach probes such as fluorophores or biotin to small bio-orthogonal chemical reporter groups introduced into macromolecules. In bio-orthogonal non-canonical amino acid tagging (BONCAT) and fluorescent non-canonical amino acid tagging (FUNCAT) proteins are metabolically labeled with a non-canonical, azide-bearing amino acid and subsequently CuAAC-clicked either to an alkyne-bearing biotin (BONCAT) for protein purification, Western blot, or mass spectrometry analyses or to an alkyne-bearing fluorophore (FUNCAT) for immunohistochemistry. In combination with mass spectrometry, these kinds of labeling and tagging strategies are a suitable option to identify and characterize specific proteomes in living organisms without the need of prior cell sorting. Here, we provide detailed protocols for FUNCAT and BONCAT click chemistry and the detection of tagged de novo synthesized proteins in Drosophila melanogaster.
Keywords: Bio-orthogonal chemical reporters (生物正交化学报告)Background
Bio-orthogonal chemical reporters are used to visualize and identify experimentally defined entities of macromolecules in living cells and organisms. Originally developed as a means for protein tracking and thus complementary to tagging by fluorescent proteins (e.g., GFP) or antibody-labeling (Tsien, 1998; Massoud and Gambhir, 2003), they can also be used to monitor glycans, lipids, nucleic acids or virus particles (Wang et al., 2003; Kho et al., 2004; Seo et al., 2004; Laughlin et al., 2008; Martin et al., 2008; Martin and Cravatt, 2009; Palaniappan and Bertozzi, 2016, Nguyen et al., 2017). Azides and alkynes are particularly well suited as bio-orthogonal chemical reporters (Prescher and Bertozzi, 2005; Dieterich, 2010) because they typically do not exist in living systems, thus precluding unwanted side reactions during tagging. At the same time, and in contrast to radioactive reporters, they allow for highly specific reactions with established tags (e.g., fluorophores or affinity tags).
The traditional copper-catalyzed azide-alkyne-cycloaddition (CuAAC [Rostovtsev et al., 2002; Tornoe et al., 2002], AAC [Huisgen, 1963]), widely known as ‘click chemistry’ is one of the principal bio-orthogonal labeling reactions as it is fast, chemically highly selective, and applicable over a wide range of biochemically compatible buffers, pH and temperature. Azide groups and alkyne groups exhibit high reactivity towards each other, and covalent bonding between them manifests in the formation of a stable triazole ring. Azide-bearing molecules can be detected using alkyne-bearing tags and vice versa. Whereas, however, alkynes can only be CuAAC-clicked, azides can also be tagged by Staudinger ligation (Saxon and Bertozzi, 2000; Kiick et al., 2002) or strain-promoted cycloaddition (e.g., Agard et al., 2004; reviewed in Lallana et al., 2011), both with a slower reaction rate (to review chemical reactions in bio-orthogonal chemistry see Prescher and Bertozzi, (2005); Ramil and Lin, (2013).
In order to tag proteomes on the basis of click chemistry, we developed bio-orthogonal non-canonical amino acid tagging (BONCAT). In the prototypic, non-cell-type- specific BONCAT approach, the non-canonical amino acid azidohomoalanine (AHA) (or homopropargylglycine [HPG]) is used to compete with methionine for tRNA loading and translational incorporation using the endogenous protein synthesis machinery. AHA bears an azide ‘handle’ for alkyne-bearing biotin tagging, allowing for subsequent protein purification, Western blot or mass spectrometry analyses (Dieterich et al., 2006). Similarly, in fluorescent non-canonical amino acid tagging (FUNCAT), AHA is clicked to an alkyne-bearing fluorophore for immunohistochemistry (Beatty et al., 2006; Dieterich et al., 2010). Most recently, this method was further advanced to enable the direct visualization of single newly synthesized proteins via proximity ligation assay (PLA) techniques (FUNCAT-PLA, tom Dieck et al., 2015). The expansion of the method to cell-type specific labeling with e.g., azidonorleucine (ANL) requires a mutation of the target organism to provide a cell-type specific MetRS capable to recognize ANL (first mutant MetRS capable to process ANL from E. Coli, Link et al., 2006, mMetRSL274G in astrocytes, Müller et al., 2015; mMetRSL274G and/or dMetRSL262G in several Drosophila tissues (Erdmann et al., 2015; Niehues et al., 2015). ANL has a longer side chain than AHA and, therefore, does not fit the binding pocket of the endogenous MetRS. Critically, this approach does not require methionine depletion as the MetRSLtoG has a higher affinity for ANL compared to AHA.
In the following, we provide detailed BONCAT, and FUNCAT protocols for cell-type specific metabolic labeling of de novo synthesized proteins with ANL in Drosophila according to Erdmann et al. (2015) and Niehues et al. (2015).
Part I: Fluorescent non-canonical amino acid tagging (FUNCAT)-Protocol
The procedure is described for preparations of larval body walls, which comprise various tissues and cell types including body wall and tracheal epithelia, sensory neurons, motor neurons, peripheral glia, and muscles. Additional tissues such as the CNS, imaginal discs or salivary glands may be left attached.
Materials and Reagents
- Tungsten needles
- Dish
- Magnetic foil
- Stainless steel pins
- 1.5 ml Eppendorf tubes
- Pasteur pipettes (Carl Roth, catalog number: 4518.1)
- 48-well plate (Sigma-Aldrich, catalog number: CLS3548)
- Microscope slides (Carl Roth, catalog number: H868.1)
- Cover slips (Carl Roth, catalog number: H874.2)
- Dissection plate: wax plate (self-made, alternatively PDMS or magnetic plates) for dissection and fixation of larval body walls
- Drosophila strains as described in Erdmann et al. (2015)
- Ultra-pure water (Carl Roth, catalog number: AE72)
- Dimethyl sulfoxide (DMSO, Sigma-Aldrich, catalog number: D8418)
- 1 mM tetramethylrhodamine- (TAMRA) alkyne-tag (Thermo Fisher Scientific, catalog number: T10183) in DMSO in 10 µl aliquots, store at -20 °C for up to 1 year, avoid repeated freezing and thawing
- 200 mM triazole ligand (TBTA; Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine) (Sigma-Aldrich, catalog number: 678937) in DMSO in 10 µl aliquots, store at -20 °C for up to 1 year, avoid repeated freezing and thawing
- 400 mM TCEP (Tris(2-carboxyethyl)phosphine hydrochloride) (Sigma-Aldrich, catalog number: C4706) in ultrapure water (prepare fresh before use)
- 200 mM CuSO4 (Sigma-Aldrich, catalog number: 1027900250) in ultrapure water (prepare fresh before use)
- VectaShieldTM Mounting Medium (Fisher Scientific, catalog number: 13273694)
- Transparent nail polish (drugstore)
- NaCl (Carl Roth, catalog number: 3957)
- KCl (Carl Roth, catalog number: 6781)
- MgCl2•6H2O (Carl Roth, catalog number: 2189)
- NaHCO3 (Carl Roth, catalog number: 8551)
- Sucrose (Carl Roth, catalog number: 4621)
- Trehalose (Carl Roth, catalog number: 5151)
- HEPES (Carl Roth, catalog number: 9105)
- CaCl2 (Carl Roth, catalog number: T885)
- Na2HPO4 (Carl Roth, catalog number: X987)
- NaH2PO4 (Carl Roth, catalog number: T879)
- PFA (Carl Roth, catalog number: 0335)
- KH2HPO4 (Carl Roth, Germany, catalog number: 3904)
- NaOH (Carl Roth, catalog number: 6771)
- HCl (Carl Roth, Germany, catalog number: 9277)
- 20% Triton® X-100 (Carl Roth, catalog number: 3051)
- Tween®-20 (Carl Roth, catalog number: 9127)
- Hemolymph-like solution, HL3 (see Recipes)
- 4% paraformaldehyde (PFA) (see Recipes)
- Phosphate buffer (PB), pH 7.2 (see Recipes)
- 1x PBS pH 7.4 or 7.8 (see Recipes)
- PBT (see Recipes)
- PBS-Tw (see Recipes)
- 50x Protease Inhibitor (PI) w/o EDTA (see Recipes)
- Homogenization buffer (see Recipes)
- 20% (w/v) SDS (see Recipes)
- PBS-SDS pH 7.8 (see Recipes)
- PBS-Igepal-630 (see Recipes)
- 2x SDS elution buffer (see Recipes)
Equipment
- Binocular (e.g., Leica, model: MZ10F)
- Forceps (Fine Science Tools, catalog number: 11255-20)
- Scissors (Fine Science Tools, catalog number: 15002-08)
- Reciprocating shaker (e.g., GFL, model 3006)
- Vortex mixer (e.g., Scientific Industries, model: Vortex-Genie 1 or 2)
- 4 °C refrigerator
- -20 °C freezer
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Marter, K., Kobler, O., Erdmann, I., Soleimanpour, E., Landgraf, P., Müller, A., Abele, J., Thomas, U. and Dieterich, D. C. (2019). Click Chemistry (CuAAC) and Detection of Tagged de novo Synthesized Proteins in Drosophila. Bio-protocol 9(2): e3142. DOI: 10.21769/BioProtoc.3142.
分类
生物化学 > 蛋白质 > 标记
细胞生物学 > 基于细胞的分析方法 > 蛋白合成
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link