- EN - English
- CN - 中文
Isolation of Microvesicles from Human Circulating Neutrophils
人循环中性粒细胞微泡的分离
(*contributed equally to this work) 发布: 2021年10月05日第11卷第19期 DOI: 10.21769/BioProtoc.3119 浏览次数: 4470
评审: Ruth A. FranklinScott McCombmohan babu
Abstract
Neutrophil-derived microvesicles (NDMVs) are liberated by neutrophils upon cell activation by molecules. Once activated, neutrophils are primarily involved in acute inflammation; however, the microvesicles they produce are largely anti-inflammatory. NDMVs have been shown to protect cartilage during inflammatory arthritis. They exert these effects by inhibiting or affecting the function of target cells, including macrophages. NDMVs have the potential to act as disease-modifying agents, especially for inflammatory diseases. This protocol describes a method using differential centrifugation to separate neutrophils from whole human blood. Subsequently, neutrophils are identified by cytospin and Wright’s staining, and then the NDMVs are isolated using differential centrifugation.
Keywords: Microvesicles (微泡)Background
Microvesicles (MVs) are a type of extracellular vesicle that can be between 100 to 1,000 nm in size (Leblanc et al., 2017; Zhan et al., 2021). They are produced by exocytic budding from the plasma membrane and can be shed from diverse cell types as a result of events such as cell activation by stimuli or apoptosis (Lima et al., 2011). Stimuli activating these events increase intracellular calcium, leading to cytoskeletal rearrangement and eventually microvesicle budding (Distler et al., 2005). MVs can carry specific sets of secreted molecules between cells, such as functional proteins, lipids, and nucleic acids including mRNAs and microRNAs, making them an important mode of intercellular communication (Leblanc et al., 2017). Through horizontal transfer of these molecules, MVs have the ability to transcriptionally regulate target cells (Greening et al., 2017). This ability, coupled with their association with a multitude of different diseases, including rheumatoid arthritis, vasculitis, and various cancers, makes them interesting targets for disease modification. MVs also exhibit superior potential as non-invasive biomarkers for certain diseases, as they are detectable in all biological fluids, including blood (both serum and plasma), urine, and saliva (Leblanc et al., 2017). Moreover, surface proteins differ based on the type of cell they are shed from (Lima et al., 2011), and both the inducer and severity of disease can alter what set of molecules MVs carry (Ridger et al., 2017).
Neutrophil-derived microvesicles (NDMVs) are liberated by neutrophils upon cell activation by molecules. Activators include N-formyl peptides, which are proteins produced by bacteria or eukaryotic mitochondria containing N-formylmethionine, and tumor necrosis factor-α (TNF-α) (Mantovani et al., 2011). When stimulated, neutrophils play a crucial role in acute inflammation; however, the microvesicles they generate are essentially anti-inflammatory (Trongtorsak et al., 2018; Zhan et al., 2021). In fact, neutrophils are part of a small group of cells whose MVs are known to promote tissue regeneration and, in some cases, repair (Rhys et al., 2018). For example, polymorphonuclear neutrophil-derived MVs (NDMVs) can protect cartilage during inflammatory arthritis (Headland et al., 2015; Topping et al., 2017). They exert these effects by inhibiting or affecting the function of target cells, including macrophages and fibroblast-like synoviocytes, which can be modified by NDMVs to display a more anti-inflammatory phenotype (Rhys et al., 2018; Zhan et al., 2021). These characteristics suggest NMDVs have potential disease-modifying effects, especially for inflammatory diseases.
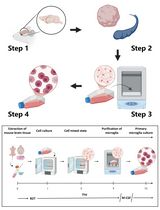
In this protocol, we describe a method using differential centrifugation to separate polymorphonuclear neutrophils from whole blood. TNF-α is added to activate these neutrophils and stimulate MV release. Finally, the MVs are isolated using differential centrifugation, and their size distribution and concentration are characterized using Nanoparticle Tracking Analysis (NTA).
Materials and Reagents
Safety blood collection set with Luer adapter (Greiner Bio-One, Vacuette®, catalog number: 450081)
4 ml NH sodium heparin blood collection tubes (Greiner Bio-One, Vacuette®, catalog number: 454051)
50 ml polypropylene centrifuge tube (Labcon, PerformRTM, catalog number: 3186-345)
15 ml polypropylene centrifuge tubes (Labcon, PerformRTM, catalog number: 3136-345)
1.5 ml polypropylene microcentrifuge tubes (Labcon, SuperSpin®, catalog number: 3016-870)
Ultracentrifuge tube (Beckman Coulter, Quick-Seal Polypropylene Tube, bell-top, catalog number: 365470)
Foil
1 ml syringe (Nipro, catalog number: JD+01D2238)
Syringe filter (Whatman, Anotop 10 syringe filter, pore size 0.02 μm, catalog number: Z747637)
ddH2O (purified by Thermo Fisher Scientific Water Purification System)
Whole human blood collected in accordance with institutional biosafety and ethics guidelines
PolymorphprepTM (Axis-Shield, catalog number: 1114683)
RPMI 1640 (Gibco, catalog number: 11530586)
Red Blood Cell Lysis Buffer (Roche, catalog number: 11 814 389 001)
Human AB serum (Thermo Fisher Scientific, Coring Human AB serum 100 ml, catalog number: MT35060CI)
Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: 60004)
Glass slide (Tharmac, catalog number: JC311-100)
Filter cards (Tharmac, catalog number: JC305-200)
Cytospin funnel (Tharmac, Single Cellfunnel, catalog number: JC304-12)
Cytoclip (Tharmac, Accessories for Cellspin, catalog number: JC302-1)
Wright’s Solution (Merck, Wright’s eosin methylene blue solution, catalog number: 101383)
Recombinant Human Tumor Necrosis Factor-α (rhTNF-α) (Biolegend, catalog number: 717904)
NaCl (Vivantis, catalog number: PC0914-1kg)
KH2PO4 (Sigma-Aldrich, catalog number: 7758-11-4)
KCl (Sigma-Aldrich, catalog number: 7447-40-7)
Na2HPO4 (Sigma-Aldrich, catalog number: 7558-79-4)
HCl (VWR International, catalog number: 7647-01-0)
Phosphate buffer saline (PBS) (see Recipes)
Equipment
Water Purification System (Thermo Scientific, Barnstead Pacific RO, catalog number: 50132389)
Pipettes: 0.5-10 μl, 10-100 μl, and 100-1,000 μl (Eppendorf, Eppendorf Research plus, catalog numbers: 3120000020, 3120000046, 3120000060)
Balance (Fisher Scientific, model: SartoriusTM MSE125P100DU)
Universal 320R centrifuge equipped with a swing-out rotor (6-place) and fixed angle rotor (24- place) (Hettich, model: UNIVERSAL 320R), Centrifuge Rotor (Hettich, model: Rotor 1619); Centrifuge Accessary (Hettich, model: accessory 1462-A); Centrifuge Rotor (Hettich, model: Rotor 1420B)
Ultracentrifuge equipped with type 100Ti fixed-angle rotor (Beckman Coulter, model: OptimaTM XPN-100), Ultracentrifuge rotor (Beckman Coulter, model: Type 100Ti Fixed angle rotor, catalog number: 363013)
Water bath equipment (Memmert, Waterbath WNB 22)
Hemocytometer (Hausser Scientific, Bright-Line Hemacytometer, catalog number: 3120)
Cytospin device (Tharmac, Cellspin I)
Light Microscope (Nikon, Upright Microscope Eclip 50i)
NanoSight NS300 (Malvern Panalytical, O-ring top-plate, 405 nm laser)
Autoclave (Tomy, catalog number: LSX-500)
Refrigerator (4°C) (Samsung, 21 cu. ft. Capacity Top Freezer Refrigerator with FlexZone)
Refrigerator (-80°C) (Thermo, standard performance ultra-low freezers)
Software
Nanosight NTA 3.1 Software Build 3.1.54 (Malvern Panalytical)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Zhan, D., McConachie, E., Edwards, S. W., Wright, H. L., Moots, R. J. and Honsawek, S. (2021). Isolation of Microvesicles from Human Circulating Neutrophils. Bio-protocol 11(19): e3119. DOI: 10.21769/BioProtoc.3119.
分类
细胞生物学 > 细胞分离和培养 > 细胞分离
免疫学 > 免疫细胞分离 > 嗜中性粒细胞
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link