- EN - English
- CN - 中文
Production, Titration and Imaging of Zika Virus in Mammalian Cells
哺乳动物细胞中寨卡病毒的制备、滴度测定和成像
(*contributed equally to this work) 发布: 2018年12月20日第8卷第24期 DOI: 10.21769/BioProtoc.3115 浏览次数: 11807
评审: David PaulVinay PanwarAnonymous reviewer(s)
Abstract
Since the outbreak of Zika virus (ZIKV) in Latin America and the US in 2016, this flavivirus has emerged as a major threat for public health. Indeed, it is now clear that ZIKV is vertically transmitted from the infected mother to the fetus and this may lead to severe neurological development defects including (but not restricted to) neonate microcephaly. Although ZIKV has been identified in the late 1940s, very little was known about its epidemiology, symptoms and molecular biology before its reemergence 60 years later. Recently, tremendous efforts have been made to develop molecular clones and tools as well as cell culture and animal models to better understand ZIKV fundamental biology and pathogenesis and to develop so-far-unavailable antiviral drugs and vaccines. This bio-protocol describes basic experimental procedures to produce ZIKV stocks and to quantify their concentration in infectious virus particles as well as to image and study this pathogen within infected cells using confocal microscopy-based imaging.
Keywords: Zika virus (寨卡病毒)Background
Zika virus (ZIKV), a mosquito-borne Flavivirus within the Flaviviridae virus family, was first isolated from a sentinel Rhesus monkey in 1947 in the Ziika Forest in Uganda and is closely related to dengue virus (DENV) (World Health Organization, 2018). Since then, it became famous in the last decade for its outbreaks in some Pacific islands (2007 and 2013) and in the Americas (2015). Symptomatic patients (25%-20% of the cases) may usually show rather mild clinical manifestations such as fevers, rashes, conjunctivitis, muscle/joint pains and/or headaches. However, since ZIKV re-emergence, severe neurological symptoms including (but not restricted to) Guillain-Barre syndrome in adults and congenitally transmitted newborn microcephaly were identified in infected individuals (Lazear and Diamond, 2016; Grubaugh et al., 2018). Unfortunately, there are currently no available treatments or vaccines against Zika virus, and this is partly due to our poor understanding of its biology.
In terms of phylogeny, there are 2 distinct lineages of Zika virus (Haddow et al., 2012). The so-called “historical” African lineage including the prototype strain MR766 and the “contemporary” Asian lineage derived from the African lineage after decades of mutations. For instance, the Asian strain H/PF/2013 is responsible for the outbreak in French Polynesia in 2013. Similarly, the virus strain responsible for the Brazilian outbreak also phylogenetically derives from the Asian lineage. Indeed, experiments in mouse and mosquito infection models support the idea that the Asian strain has acquired several key mutations which led to microcephaly in infected newborns in Brazil (Yuan et al., 2017) and to enhanced viral infectivity in the arthropod transmission vector Aedes aegypti (Liu et al., 2017).
Upon entry in the host cell, the viral genome, a positive single-stranded RNA, is translated into a single polyprotein at the endoplasmic reticulum (ER), which is subsequently cleaved into 3 structural proteins and 7 non-structural proteins by host and viral proteases. After replication, the genome is encapsidated into neosynthesized virions which are subsequently released from the cell (Neufeldt et al., 2018). Like all other tested flaviviruses, ZIKV remodels the endomembranes of the infected cell to generate a cytoplasmic environment which is favorable to ZIKV life cycle (Cortese et al., 2017). These membranous compartments, called viral replication factories are all ER-derived and can be sub-divided into three classes of ultrastructures (Cortese et al., 2017): 1. vesicle packets, which are ER invaginations believed to be the site of viral RNA replication, 2. virus bags in which assembled immature viruses accumulate in regular arrays, and 3. convoluted membranes whose roles remain unclear although it was proposed for DENV that they modulate host processes for the benefit of replication (Chatel-Chaix et al., 2016).
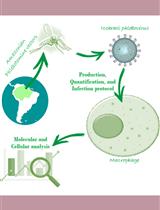
Before 2016, little was known about the general biology of ZIKV, and most of our knowledge relied on a direct transposition of fundamental discoveries about other flaviviruses closely related to this pathogen like DENV. However, it remains unclear what makes ZIKV so unique in terms of neuropathogenesis as compared to the other members of the Flavivirus genus. Hence, since ZIKV re-emergence and the recent outbreaks, the scientific community around the world has begun to decipher the mysteries surrounding this virus and to develop tools such as molecular clones as well as cell culture and animal models (Schwarz et al., 2016; Shan et al., 2016; Xie et al., 2016; Morrison and Diamond, 2017; Mutso et al., 2017; Munster et al., 2018). Knowing how to culture ZIKV and to measure its infectivity constitutes key methods to perform any descriptive or functional studies about this virus both in cellulo and in vivo. Moreover, given that Asian and African lineages may differ in terms of symptom severity in infected mouse fetuses (Cugola et al., 2016), it is sometimes relevant to compare model strains of these two lineages. In this bio-protocol, we describe basic cell culture methods to produce ZIKV stocks from Asian and African lineages and to quantify their concentration in infectious virus particles (more generally referred to as “titer”) as well as to image this pathogen inside infected cells using immunofluorescence-based microscopy.
Materials and Reagents
- Materials
- 1.5 ml microtubes (Ultident Scientific, catalog number: 87-B150-C)
- 10 cm cell culture dishes (Falcon, Fisher Scientific, catalog number: 08-772-E)
- 15 cm cell culture dishes (Falcon, Fisher Scientific, catalog number: 08-772-6)
- 24-well polystyrene culture plates (Corning Life Sciences, Fisher Scientific, catalog number: 08-772-1)
- MCE 0.45 μm filters, 30 mm diameter (Ultident Scientific, catalog number: 229753)
- 20 ml sterile syringes (Ultident Scientific, catalog number: BD-302830)
- 15 ml sterile conical tubes (Falcon, Fisher Scientific, catalog number: 352096)
- Coverslips No. 1 (diameter: 12 mm, thickness: 0.13 to 0.17 mm), sterilized by autoclaving (Fisher Scientific, catalog number: 12-545-80)
- Microscope glass slides, frosted clear glass 26 mm x 76 mm, 1-1.2 mm thick (Ultident Scientific, catalog number: 170-8105-W)
- Absorbent paper
- Parafilm
- Aluminum foil
- Viruses
- ZIKV MR766 (African lineage) (EVAg, catalog number: 001v-EVA143) (under material and transfer agreement. Passage history: P5. Store original desiccated stocks at -80 °C)
- ZIKV H/PF/2013 (Asian lineage) (EVAg, catalog number: 001v-EVA1545) (under material and transfer agreement. Passage history: P6. Store original desiccated stocks at -80 °C)
- Cell lines
- Vero E6 monkey epithelial cells (a kind gift from Drs. Tom Hobman and Anil Kumar, University of Alberta) (ATCC, catalog number: CRL-1586)
- Huh7 hepatocarcinoma cells (a kind gift from Dr. Patrick Labonté, INRS) (Creative Bioarray, catalog number: CSC-C9441L)
Alternatively, this cell line is typically available from most laboratories working on hepatitis C virus or DENV.
- Reagents
- UltraPure distilled water (Life Technologies, catalog number: 10977-015)
- Dulbecco's modified Eagle medium (DMEM) (Life Technologies, catalog number: 111965-092)
- Fetal bovine serum (FBS) performance (Wisent, catalog number: 098150)
- Penicillin-streptomycin (Life Technologies, catalog number: 15140-122)
- MEM non essential amino acids solution (100x) (Life Technologies, catalog number: 11140-050)
- Phosphate buffered saline (PBS) (Life Technologies, catalog number: 14190-144)
- 1 M HEPES buffer, pH range: 7.2-7.5 (Life Technologies, catalog number: 15630-080)
- 0.25% Trypsin-EDTA (Life Technologies, catalog number: 25200-072)
- Minimum Essential Medium (MEM) with L-Glutamine (Life Technologies, catalog number: 11095-080)
- Carboxymethylcellulose (CMC) sodium salt, medium viscosity (Sigma-Aldrich, catalog number: 21902-100G )
- 37% formaldehyde (Fisher Scientific, catalog number: BP531-500)
- Crystal violet (Fisher Scientific, catalog number: C581-100)
- 95% ethanol (Commercial Alcohols, catalog number: 1011C)
- Triton X-100 (Mallinktrot, catalog number: 3555)
- 4% paraformaldehyde (Sigma-Aldrich, catalog number: P6148-1KG)
- Normal goat serum (Thermo-Fisher, catalog number: 01-6201)
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A-9647)
- Sodium azide (Fisher Scientific, catalog number: BP922I-500)
- Rabbit polyclonal anti-ZIKV NS4B (GeneTex, catalog number: GTX133311; dilution 1:200)
- Mouse monoclonal anti-dsRNA, clone J2 (Scicons, catalog number: 10010200; dilution 1:400)
- Rabbit polyclonal anti-DENV NS4B (GeneTex, catalog number: GTX124250; dilution 1:1,000)
- Mouse monoclonal anti-DENV NS3, clone GT2811 (GeneTex, catalog number: GTX629477; dilution 1:100)
- Mouse monoclononal panflaviviral anti-E, clone 4G2 (Sigma-Aldrich, catalog number: MAB10216, dilution 1:200)
- Goat anti-Rabbit AlexaFluor 488 (Life Technologies, catalog number: A11034)
- Goat anti-Mouse AlexaFluor 568 (Life Technologies, catalog number: A11004)
- 4',6-Diamidino-2-Phenylindole (DAPI) (Life Technologies, catalog number: D1306)
- Fluoromount G (Southern Biotech, catalog number: 0100-01)
- Complete DMEM (see Recipes)
- Plaquing medium (MEM-CMC) (see Recipes)
- Formaldehyde fixative (see Recipes)
- Paraformaldehyde fixative (see Recipes)
- Crystal violet staining solution (see Recipes)
- Triton X-100 permeabilization solution (see Recipes)
- BSA Blocking solution (see Recipes)
Equipment
- -80 °C freezer
- Vortexer
- Pipette
- Beaker
- CO2 incubator
- Hemacytometer
- Magnetic stirrer
- BSL2 cell culture cabinet
- 2D rocking platform shaker
- Zeiss LSM780 confocal microscope
- Chemical hood
- Tweezers
- 4 °C refrigerator
- Autoclavable glass bottle
- Autoclave
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Freppel, W., Mazeaud, C. and Chatel-Chaix, L. (2018). Production, Titration and Imaging of Zika Virus in Mammalian Cells. Bio-protocol 8(24): e3115. DOI: 10.21769/BioProtoc.3115.
分类
微生物学 > 微生物细胞生物学 > 病毒繁殖
细胞生物学 > 细胞分离和培养 > 病毒分离
分子生物学 > 蛋白质 > 检测
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link