- EN - English
- CN - 中文
Papain-based Single Cell Isolation of Primary Murine Brain Endothelial Cells Using Flow Cytometry
利用流式细胞技术进行原代小鼠脑内皮细胞的基于木瓜蛋白酶的单细胞分离
发布: 2018年11月20日第8卷第22期 DOI: 10.21769/BioProtoc.3091 浏览次数: 11782
评审: Pengpeng LiRAVI THAKURPatrick Ovando-Roche

相关实验方案
来自骨髓增生性肿瘤患者的造血祖细胞的血小板生成素不依赖性巨核细胞分化
Chloe A. L. Thompson-Peach [...] Daniel Thomas
2023年01月20日 2302 阅读
Abstract
Brain endothelial cells (BECs) form the integral component of the blood-brain barrier (BBB) which separates the systemic milieu from the brain parenchyma and protects the brain from pathogens and circulating factors. In order to study BEC biology, it was of particular interest to establish a method that enables researchers to investigate and understand the underlying molecular mechanisms regulating their function during homeostasis, aging and disease. Furthermore, due to the heterogeneity of the cerebrovasculature and different vessel types that comprise the BBB, it is of particular interest to isolate primary BECs for single cell analysis from various subregions of the brain, such as the neurogenic and highly vascularized hippocampus and to enrich for specific vessel types. In the past, approaches to isolate endothelial cells were dependent on transgenic mice and often resulted in insufficiently pure cell populations and poor yield. This protocol describes a technique that allows single-cell isolation of highly pure brain endothelial cell populations using fluorescence activated cell sorting (FACS). Briefly, after perfusion and careful removal of the meninges, and dissection of the cortex/hippocampus, the brain tissue is mechanically homogenized and enzymatically digested resulting in a single cell suspension. Cells are stained with fluorochrome-conjugated antibodies identifying CD31+ brain endothelial cells, as well as CD45+CD11b+ myeloid cells for exclusion. Using flow cytometry, cell populations are separated and CD31+BECs are sorted in bulk into RNA later or as single cells directly into either RNA lysis buffer for single or bulk RNA-Seq analyses. The protocol does not require the expression of a transgene to label brain endothelial cells and thus, may be applied to any mouse model. In our hands, the protocol has been highly reproducible with an average yield of 1 x 105 cells isolated from an adult mouse cortex/hippocampus.
Keywords: Brain endothelial cells (脑内皮细胞)Background
The Blood-brain barrier (BBB) is a complex vascular structure that separates and protects the brain from systemic circulating factors and immune cells while allowing selective transport of critical nutrients. This highly specialized vasculature regulates selective transport of metabolites across the barrier. BECs also form a central component of the neurovascular unit (NVU), with close association and crosstalk with various cell types in the brain including neural precursor cells, microglia and the brain-resident immune cells. The BBB not only limits the passage of ions and other molecules such as glucose but also prevents the uncontrolled exchange of toxins, bacteria, viruses and cells between the blood and the brain parenchyma (Abbott et al., 2010). Nutrient-rich, oxygenated blood is pumped into the brain through cerebral arterial BECs (arteries and arterioles), which are protected and supported by smooth muscle cells (SMCs) that cover the endothelium and form a basement membrane layered by astrocytic end-feet of the brain parenchyma (Figure 1). The blood is transferred to highly specialized capillaries, which are comprised of BECs that form unique tight junctions and are wrapped by pericytes (Peric.) within the endothelial basement membrane, which is then covered by astrocytic end-feet. BBB capillaries are the site of controlled transport of fluids and solutes into the CNS. Immuno-surveillance and occasional extravasation of leukocytes (Leuk.) into the CNS parenchyma occurs at the level of postcapillary venous cells (venules and veins) the vascular segments into which blood flows after passing through the capillaries. Postcapillary Venules contain enlarged perivascular space between the endothelial and astrocytic basement membranes where occasional immune cells can reside (Banks, 2016; Engelhardt et al., 2017).
The outlined fluorescent activated cell sorting (FACS) single cell isolation method is based on previously described procedure for BEC isolation (Tam et al., 2012), that have been further developed and modified in context of a study aiming to determine molecular changes occurring in brain endothelial cells during aging and exposure to aged blood (Yousef et al., 2018). A schematic of the FACS procedure for BEC isolation and an example of cell gating are shown in Figure 2. Aging results in the upregulation of chronic inflammatory processes involving endothelial cell activation that contributes to reduced neural precursor cell activity and increased neuroinflammation in the aging brain (Yousef et al., 2018). The technique was successfully applied in a collaborative effort to investigating the effects of an aged systemic milieu on hippocampal neurogenesis and microglia activation (Yousef et al., 2018). The study highlights the role of the vascular cell adhesion molecule 1 (VCAM1) as a negative regulator of adult neurogenesis and inducer of microglial activity. VCAM1 is only expressed in a very small subpopulation of cells under baseline conditions and thus, was not sufficiently immunolabeled using conventional two-step antibody staining to perform FACS analysis. To that end, mice were stimulated systemically with Lipopolysaccharide (LPS) from Salmonella typhimurium to increase VCAM1 expression. The mice were then retro-orbitally injected with fluorescently labeled anti-mouse VCAM1, which resulted in a reliable detection of the VCAM1 positive brain endothelial cell subpopulation. The CD31+VCAM1+ BECs in LPS-stimulated mice could be used to set positive and negative gates in the flow sorter to quantitatively assess CD31+VCAM1+ expression in normal young and aged mice, or young mice treated with young or aged plasma and to isolate this rare subpopulation (Yousef et al., 2018; Figure 3). Additionally, VCAM1 is enriched in arterial and venous BECs and can be used to enrich and study these two vessel segmental populations (Vanlandewijck et al., 2018; Yousef et al., 2018).
In the last decade, several protocols for brain endothelial cell isolation have been employed, which tend to use transgenic endothelial cells labeled with fluorescent proteins such as GFP (Daneman et al., 2010; Vanlandewijck et al., 2018). Because these techniques are dependent on transgenic endothelial cell markers, they may not easily be applied to diseased or normal aged mouse models. This protocol allows the isolation of highly pure brain endothelial cell population from any mouse strain or murine animal model using papain-based enzymatic digestion using the commercial neural dissociation kit (MACS Miltenyi Biotec, Miltenyi). 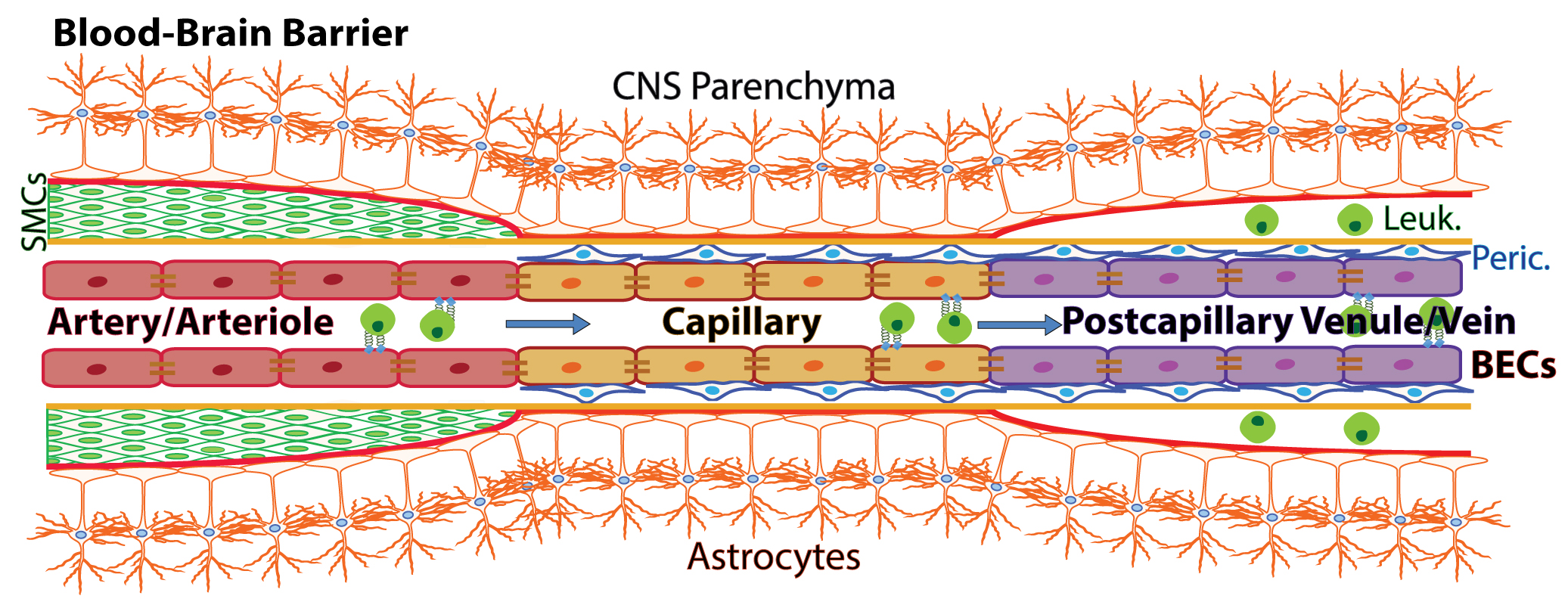
Figure 1. Schematic of the blood-brain barrier. Nutrient-rich, oxygenated blood is pumped into the brain through cerebral arterial BECs (arteries and arterioles), which are protected and supported by smooth muscle cells (SMCs) that cover the endothelium and form a basement membrane layered by astrocytic end-feet of the brain parenchyma. The blood is transferred to highly specialized capillaries, which are comprised of BECs that form unique tight junctions and are wrapped by pericytes (Peric.) within the endothelial basement membrane, which is then covered by astrocytic end-feet. BBB capillaries are the site of controlled transport of fluids and solutes into the CNS. Immuno-surveillance and occasional extravasation of leukocytes (Leuk.) into the CNS parenchyma occurs at the level of postcapillary venous cells (venules and veins) the vascular segments into which blood flows after passing through the capillaries. Postcapillary Venules contain enlarged perivascular space between the endothelial and astrocytic basement membranes where occasional immune cells can reside (Banks, 2016; Engelhardt et al., 2017). (Adopt from Yousef et al. [2018] Figure 2a)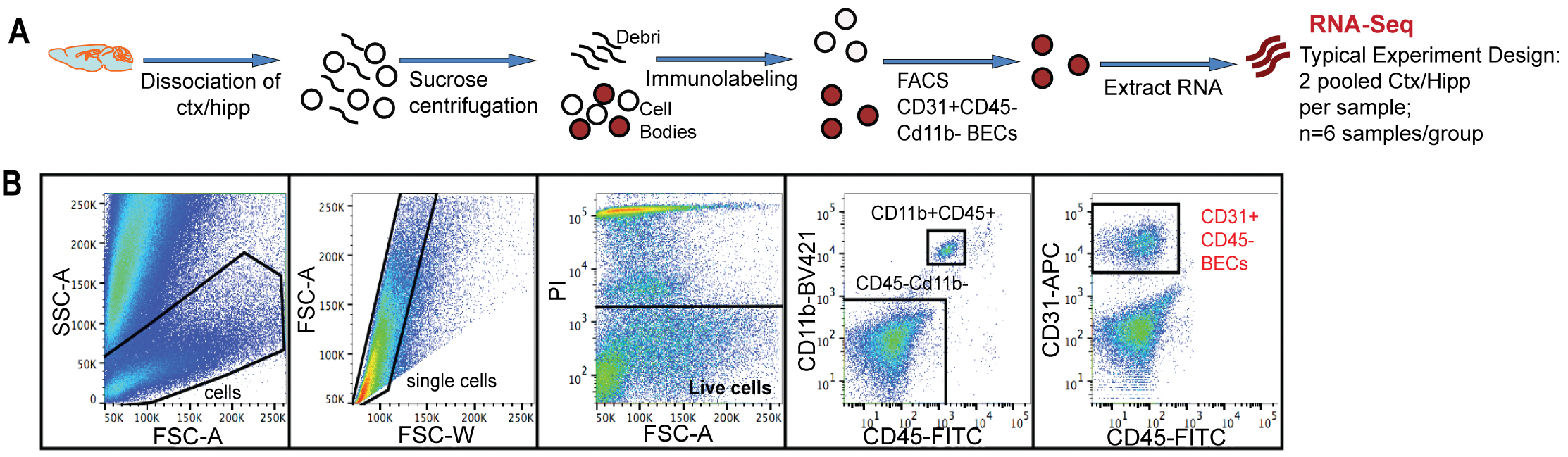
Figure 2. Mouse Brain endothelial cell isolation through flow cytometry. A. Schematic of flow sorting of CD31+CD45- BECs from mouse cortex and hippocampi. Each isolated RNA sample is a pool of BECs from 2 mouse brains. B. FACS gating strategy to isolate single BECs. PI+ dead cells were excluded. CD11b+ and CD45+ cells were gated to exclude monocytes/macrophages and microglia. CD31+Cd11b-CD45- cells were defined as the BEC population. (Adopt from Yousef et al. [2018] Supplemental Figure 1a-b)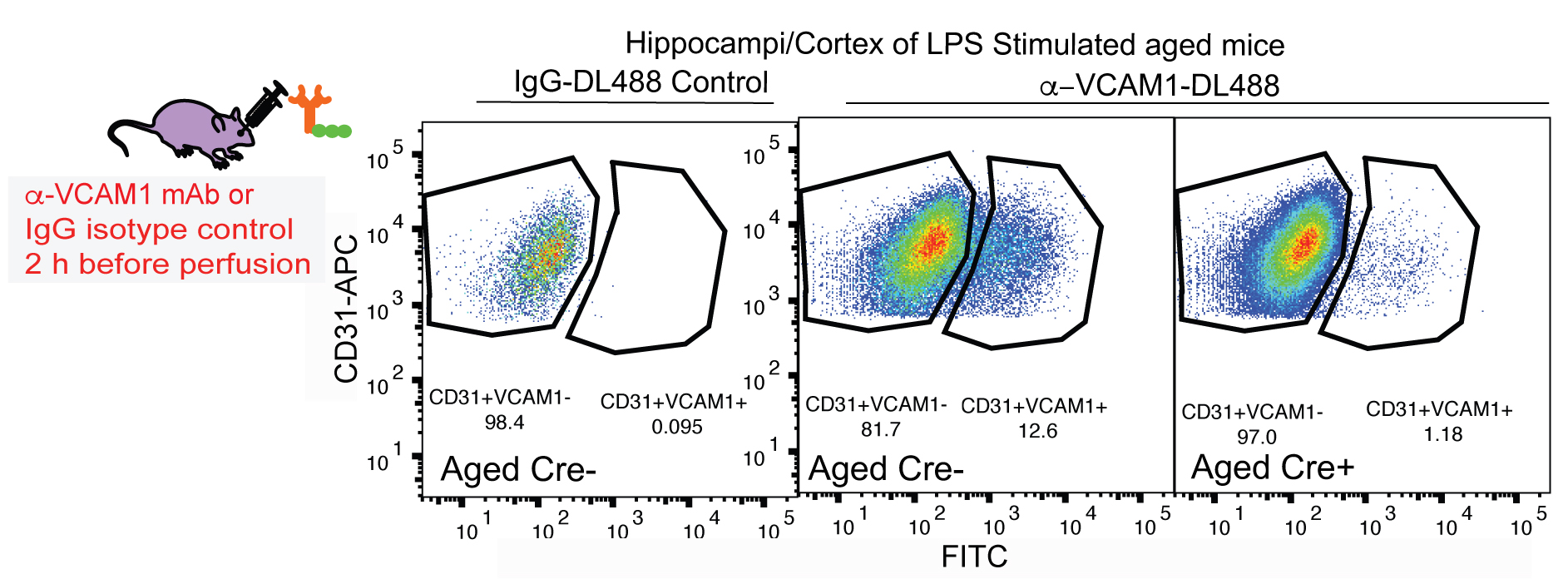
Figure 3. Identification of VCAM1+CD31+ BECs through flow cytometry. Vcam1fl/fl Slco1c1-CreERT2+/- mice (Cre+) received tamoxifen (100 mg/kg; i.p.) once daily for 5 days. After a 3-day resting period, mice were treated with LPS for 16 h and 2 h before perfusion (1 mg/kg, i.p.) and fluorescently labeled anti-VCAM1 mAb (100 µg, r.o.) for 2 h prior to cell isolation and flow cytometry analysis. Gating plots of CD31+VCAM1+ cells isolated from Gating plots are of tamoxifen-treated, LPS stimulated aged (19-month-old) Slco1c1-CreERT2+/--Vcam1fl/fl (Cre+) and littermate control mice lacking a copy of the Cre gene (Cre-), injected with fluorescently-tagged DL488 anti-VCAM1 mAb or IgG-DL488 isotype control (r.o.) 2 h before sacrifice.
Materials and Reagents
- Pipette tips must be sterile and low retention
- Whatman paper: GE Healthcare Whatman Quantitative Filter Paper Grade 40 (Fisher Scientific, GE Healthcare, catalog number: 09-927-541)
- Thermo Scientific Nalgene Rapid-Flow Sterile Disposable Filter Units with PES Membrane (Thermo Fisher, catalog number: 569-0020)
- 15 ml and 50 ml tubes (Corning, catalog numbers: 430790 and 430828)
- 1.5 ml and 2.0 ml Eppendorf Tubes Protein Low-Binding (Eppendorf, catalog numbers: 022431081 and 022431102)
- FACS Tubes: 5 ml Polystyrene Round-bottom tube with 40 μm Cell Strainer tops (Corning, Falcon®, catalog number: 352235)
- 70 μm cell strainer (Fisher Scientific, catalog number: 22-363-548)
- 40 μm cell strainer caps
- Razor blades
- Plate
- Dry ice
- 2-Mercaptoethanol (BME) (Sigma-Aldrich, catalog number: M6250-100)
- RNasin Ribonuclease Inhibitors (Promega, catalog number: N2115)
- RNA Later (Life Technologies, catalog number: AM7020)
- Miltenyi Neural Dissociation Kit (NDK) (Papain) (MACS Miltenyi Biotec, Miltenyi, catalog number: 130-092-628)
Note: Store at 2-8 °C, Shelf life: 24 months from the date of manufacture. - Fc Block: Purified Rat Anti-Mouse CD16/CD32 (Fisher Scientific, BD Pharmingen, catalog number: BDB553142) (Store at 4 °C)
- CD31-APC: APC Rat Anti-Mouse CD31 (BD Biosciences, catalog number: 551262) (Store at 2-8 °C)
- CD45-APC/Cy7: APC/Cy7 Rat Anti-Mouse CD45 (BioLegend, catalog number: 103115) (Store at 2-8 °C)
- Or CD45-FITC: FITC Rat Anti-Mouse CD45 (BD Pharmingen, catalog number: 553080, Clone 30-F11)
- CD11b-BV421: Brilliant Violet 421 Rat Anti-Mouse CD11b (BioLegend, catalog number: 101235) (Store at 2-8 °C)
- Propidium Iodide (PI): Propidium Iodide Solution (Sigma-Aldrich, catalog number: P4864) (Store at 2-8 °C)
- RNeasy Plus Micro kit (QIAGEN, catalog number: 74034)
- Optional: Rat monoclonal anti-VCAM1 (Clone M/K-2.7, Bioxell, catalog number: BE0027)
- Optional: Rat IgG1 Isotype antibody (Clone HRPN, Bioxell, catalog number: BE0088)
- Optional: DyLightTM Antibody Labeling Kit (DyLightTM 488, Thermo Scientific, catalog number: 53025)
- Modified DPBS (mDPBS) (see Recipes)
Note: Sterile filter solution, Store at 2-8 °C, Shelf life: 36 months from the date of manufacture.- Dulbecco’s Phosphate-buffered Saline (DPBS) with Calcium and Magnesium (Thermo Fisher, GibcoTM, catalog number: 14040-133)
Note: Store at 2-8 °C, Shelf life: 36 months from the date of manufacture. - Glucose (1,000 mg/L) (Fisher Scientific, ACROS Organics, catalog number: 41095-5000)
- Sodium Pyruvate (30 mg/L) (Thermo Fisher, GibcoTM, catalog number: 11360-070)
- Dulbecco’s Phosphate-buffered Saline (DPBS) with Calcium and Magnesium (Thermo Fisher, GibcoTM, catalog number: 14040-133)
- FACS buffer (see Recipes)
Note: Sterile Filter solution, Store at 2-8 °C, Shelf life: 3 months from the date of manufacture.- Modified DPBS
- 0.5% BSA (Fisher Scientific, catalog number: BP1600-100)
- 2 mM EDTA (Thermo Fisher, InvitrogenTM, catalog number: AM9912)
- 0.9 M Sucrose (see Recipes)
Sucrose in DPBS (Fisher Scientific, catalog number: BP220-10)
Note: Sterile Filter solution, Store at 2-8 °C, Shelf life: 3 months from the date of manufacture.
Equipment
- Graduated cylinder
- -80 °C freezer
- Shaker
- Centrifuge (Eppendorf, model: 5810R, catalog number: 022625004)
- Warm Water Bath (preheated at 37 °C)
- Multi-Purpose Rotator (Thermo Fisher, Lab Rotator, catalog number: 2309-1CEQ)
- BD FACSAriaTM II or III cell sorter (BD Biosciences)
- Microscissors, fine forceps
- Agilent 2100 Bioanalyzer (Agilent Technologies)
Software
- BD FACSDIVATM SOFTWARE (BD Biosciences, version: V8.0.1)
- FlowJoTM (© FlowJo, LLC, version: 9.9.4 or higher)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Yousef, H., Czupalla, C. J., Lee, D., Butcher, E. C. and Wyss-Coray, T. (2018). Papain-based Single Cell Isolation of Primary Murine Brain Endothelial Cells Using Flow Cytometry. Bio-protocol 8(22): e3091. DOI: 10.21769/BioProtoc.3091.
分类
神经科学 > 细胞机理 > 细胞分离和培养
细胞生物学 > 细胞分离和培养 > 细胞分离 > 流式细胞术
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link