- EN - English
- CN - 中文
Purification of Soluble Recombinant Human Tau Protein from Bacteria Using Double-tag Affinity Purification
双标签亲和纯化法从细菌中纯化人可溶重组Tau蛋白
发布: 2018年11月20日第8卷第22期 DOI: 10.21769/BioProtoc.3043 浏览次数: 9084
评审: Laia ArmengotAnonymous reviewer(s)
Abstract
Dysfunction of the microtubule-associated protein Tau (encoded by the MAPT gene) has been implicated in more than twenty neurodegenerative diseases, including Alzheimer’s. As such, the physiological and disease-relevant functions of Tau have garnered great interest in the research community. One barrier hampering investigations into the functions of Tau and the generation of pharmacological agents targeting Tau has been the difficulty of obtaining soluble Tau protein in purified form. Here, we describe a protocol that uses dual affinity tag purification to selectively purify soluble recombinant Tau protein from bacteria that is functionally active for downstream applications including immunization, microtubule binding assays, and protein-protein interaction studies.
Keywords: Tau (Tau蛋白)Background
Tau is traditionally defined as a microtubule binding protein; however, in human diseases Tau can dissociate from axonal microtubules and mislocalize to other neuronal compartments including the soma, dendrites, and synapses, where interactions with non-microtubule proteins and structures drive neuronal dysfunction (Iqbal et al., 2016; Wang and Mandelkow, 2016; Zhou et al., 2017; McInnes et al., 2018). Although Tau aggregates in the form of neurofibrillary tangles are commonly found in post-mortem diseased brain tissue, studies suggest that soluble Tau, not aggregated Tau, is largely responsible for neuronal dysfunction (Crimins et al., 2012; Polydoro et al., 2014; Koss et al., 2016). As such, investigating the soluble functions of Tau in disease, such as identifying protein-protein interaction partners, is therefore of critical importance to target Tau dysfunction.
Purifying soluble Tau protein has been challenging since many ectopically expressed recombinant proteins aggregate into insoluble inclusion bodies in bacteria. Tau furthermore contains aggregation-prone motifs, making purifying recombinant Tau even more difficult. We have therefore developed a protocol that overcomes these obstacles by optimizing two key aspects of this process: first, we express Tau as a fusion protein with an N-terminal Glutathione S-transferase (GST) tag to enhance solubility of the protein, and utilize a mild induction paradigm to minimize the formation of insoluble Tau aggregates. We chose GST over other fusion proteins because it is relatively small, robustly enhances protein solubility and stability, can be easily cleaved, and is relatively inexpensive to purify. Second, our purification protocol utilizes dual affinity tag purification of both an N-terminal GST tag and a C-terminal 8xHis tag (Figure 1); because this purification protocol requires both N- and C-terminal tags to be available to bind affinity resin, the protocol therefore specifically enriches for soluble, non-aggregated protein (in the event of aggregation, at least one of these epitopes is hidden within the aggregate). Following the first purification step against the N-terminal GST tag, the GST fusion protein is cleaved using PreScission protease, resulting in a final product of purified Tau protein that contains only an additional eight histidine residues (8xHis tag) at its C-terminal domain that can also be used for downstream applications including pull-down experiments or detection using anti-His antibodies. This method is versatile and can be easily modified to purify different isoforms of Tau, Tau carrying point mutations, or Tau truncations by altering the pGEX_GST-Tau0N4R-8xHis plasmid using site-directed mutagenesis or traditional cloning methods. We have successfully utilized this protocol to produce various isoforms of Tau, including with point mutations or domain truncations, for use in in vitro binding assays with synaptic vesicles (Zhou et al., 2017) and as bait in protein-protein interaction studies (McInnes et al., 2018).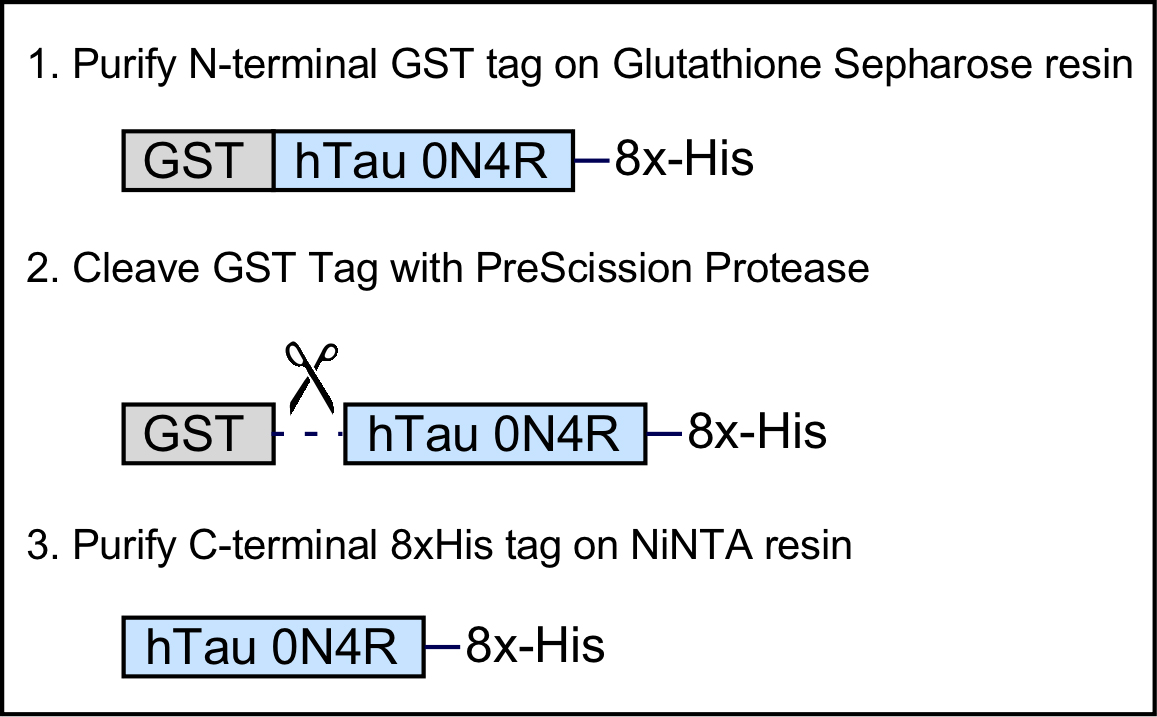
Figure 1. Schematic overview of the recombinant GST-Tau-8xHis fusion protein and purification steps
Materials and Reagents
- 1 ml micropipette tips
- Microcentrifuge tubes
- Amicon Ultra-0.5 Centrifugal Filter Unit with Ultracel-10 membrane (Merck, catalog number: UFC501024 )
- Plasmid pGEX_GST-Tau0N4R-8xHis
Note: This plasmid can be generated by cloning a cDNA encoding human MAPT into a pGEX-6P-1 vector backbone (such as the BamHI/EcoRI restriction sites of Addgene plasmid number 46408). The cDNA should be at downstream of N-terminal GST fusion with PreScission Protease cleavage site. The 8xHis tag can be inserted into the 3’ primer used to amplify the hTau cDNA. In this protocol, we use human MAPT cDNA corresponding to the 0N4R isoform. - RosettaTM bacteria cells (Merck, catalog number: 70953 )
- LB Broth (Sigma-Aldrich, catalog number: L3522 )
- Ampicillin (Sigma-Aldrich, catalog number: A9393 )
- Chloramphenicol (Sigma-Aldrich, catalog number: C0378 )
- Anti-His antibody (such as Thermo Fisher Scientific, catalog number: 37-2900 ) or anti-Tau antibody (such as Agilent Technologies, DAKO, catalog number: A0024 )
- Isopropyl β-D-thiogalactoside (IPTG) (Sigma-Aldrich, catalog number: I6758 )
Note: Prepare 100 mM stock in sterile H2O and freeze aliquots at -20 °C. Do not re-freeze after thawing. - Glutathione Sepharose 4B (GE Healthcare, catalog number: 17075601 )
- Complete protease inhibitor tablets, EDTA-free (Roche Diagnostics, catalog number: 11873580001 )
- Phenylmethanesulfonyl fluoride (PMSF), 100 mM solution (Sigma-Aldrich, catalog number: 93482 )
- Benzonase Nuclease, 250 units/μl (Sigma-Aldrich, catalog number: E1014 )
- PreScission Protease, 2,000 units/ml (GE Healthcare, catalog number: 27084301 )
Note: Upon receiving stock, thaw on ice and prepare ~10 μl aliquots and freeze at -20 °C. Do not re-freeze aliquots after thawing. - Ni-NTA ProfinityTM IMAC Resin, Ni-charged (Bio-Rad Laboratories, catalog number: 1560131 )
- Quick StartTM Bradford Protein Assay (Bio-Rad Laboratories, catalog number: 5000201 )
- PageBlueTM Protein Staining Solution (Thermo Fisher Scientific, catalog number: 24620 )
- Triton X-100 (Sigma-Aldrich, catalog number: X100 )
- Glycerol (Sigma-Aldrich, catalog number: G5516 )
- Lysozyme (Sigma-Aldrich, catalog number: L6876 )
- Sodium phosphate dibasic anhydrous (Na2HPO4) (Sigma-Aldrich, catalog number: 795410 )
- DL-Dithiothreitol (DTT), 1 M stock solution in H2O (Sigma-Aldrich, catalog number: 646563 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S3014 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E6758 )
- Tween-20 (Sigma-Aldrich, catalog number: P1379 )
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: S8045 )
- Imidazole (Sigma-Aldrich, catalog number: 792527 )
- Bacteria lysis buffer (see Recipes)
- Phosphate buffered saline (PBS) (see Recipes)
- PBS + 250 mM NaCl (see Recipes)
- PreScission protease cleavage buffer (see Recipes)
- Ni-NTA wash buffer (see Recipes)
- Ni-NTA elution buffer (see Recipes)
Equipment
- Pipettes
- Table-top microcentrifuge capable of speeds up to 16,000 x g
- Microplate reader or spectrophotometer capable of absorbance readings at 600 nm for bacterial density measurements and at 595 nm for Bradford protein assay
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
McInnes, J., Zhou, L. and Verstreken, P. (2018). Purification of Soluble Recombinant Human Tau Protein from Bacteria Using Double-tag Affinity Purification. Bio-protocol 8(22): e3043. DOI: 10.21769/BioProtoc.3043.
分类
神经科学 > 细胞机理 > 蛋白质分离
神经科学 > 神经系统疾病 > 细胞机制
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link