- EN - English
- CN - 中文
Fluorescence Titrations to Determine the Binding Affinity of Cyclic Nucleotides to SthK Ion Channels
荧光滴定法测定环核苷酸与SthK离子通道的结合亲和力
发布: 2018年10月05日第8卷第19期 DOI: 10.21769/BioProtoc.3041 浏览次数: 9274
评审: Vamseedhar RayaproluMalgorzata LichockaQiangjun Zhou

相关实验方案
膜蛋白–配体相互作用研究的计算流程:以含 α5 亚基的 GABA(A) 受体为例
Syarifah Maisarah Sayed Mohamad [...] Ahmad Tarmizi Che Has
2025年11月20日 1947 阅读
Abstract
The cyclic-nucleotide modulated ion channel family includes cyclic nucleotide-gated (CNG) and hyperpolarization-activated and cyclic nucleotide-modulated (HCN) channels, which play essential roles in visual and olfactory signaling and the heart pacemaking activity. Functionally, these channels have been extensively characterized by electrophysiological techniques from protein heterologously expressed in Xenopus oocytes and mammalian cells. On the other hand, expression and purification of these proteins for biophysical and structural analyses in vitro is problematic and expensive and, accordingly, only limited information on the purified channels is available in the literature. Here we describe a protocol for binding studies of fluorescently labeled cyclic nucleotides to a homologue of eukaryotic CNG channels. Furthermore, we describe how to directly probe binding of unlabeled cyclic nucleotides in a competition assay. The use of fluorescence as a sensitive probe for ligand binding reduces the amount of protein needed and enables fast and easy measurements using standard laboratory equipment.
Keywords: Fluorescence titration (荧光滴定法)Background
Understanding the function of a protein in molecular detail requires extensive microscopic characterization. For ligand-gated ion channels different assays are needed to gain information about the specific interaction of the protein with the ligand, the communication between the ligand binding site and the pore, as well as channel-specific characteristics such as ionic throughput and inactivation or desensitization properties. Together with structural data for channel conformations corresponding to various functional states, this allows the development of a complete mechanistic description of channel function and regulation. Cyclic nucleotide-gated (CNG) ion channels are tetrameric potassium channels that are of particular interest due to their function in olfactory and visual signaling cascades (Kaupp and Seifert, 2002; Craven and Zagotta, 2006). However, there is only very limited data available on purified CNG channels under defined conditions, mostly from single molecule force spectroscopy (Higgins et al., 2002; Maity et al., 2015; Goldschen-Ohm et al., 2016; Mazzolini et al., 2018). Electrophysiological characterization of CNG channels expressed in Xenopus oocytes or mammalian cells (Biel et al., 1993 and 1994; Baumann et al., 1994; Weyand et al., 1994; Yu et al., 1996; Zagotta and Siegelbaum, 1996), shows that they are activated by micromolar concentrations of cyclic nucleotides (cNMP), but cAMP and cGMP can act differentially on different channel subtypes. High resolution structures of a few cyclic nucleotide-modulated channels have recently been solved (James et al., 2017; Lee and MacKinnon, 2017; Li et al., 2017; Rheinberger et al., 2018), highlighting the need for biophysical assays to characterize these channels in order to gain a better understanding of the molecular interactions during gating and regulation.
Recently, we introduced SthK, a bacterial homologue of eukaryotic CNG channels, as a model to analyze the allosteric regulation of these channels in vitro (Schmidpeter et al., 2018). One interesting observation was that cAMP and cGMP activate SthK with considerably different efficacies but interact with the protein with very similar affinities. We used fluorescently labeled cyclic nucleotides (Figure 1) to measure the binding affinity of these ligands to full-length SthK channels. The fluorescence of the NBD moieties increases upon interaction with a binding partner and thus directly reports the complex formation with SthK. To probe direct binding of unlabeled nucleotides to the channel we employed fluorescence-based competition assays. In the following we will provide detailed protocols for both experiments which can be performed in any protein-biochemistry laboratory using standard equipment and are applicable to protein-ligand interactions in general. Furthermore, this assay can be adapted to a plate-reader format which might increase the throughput and at the same time account for possible pipetting errors.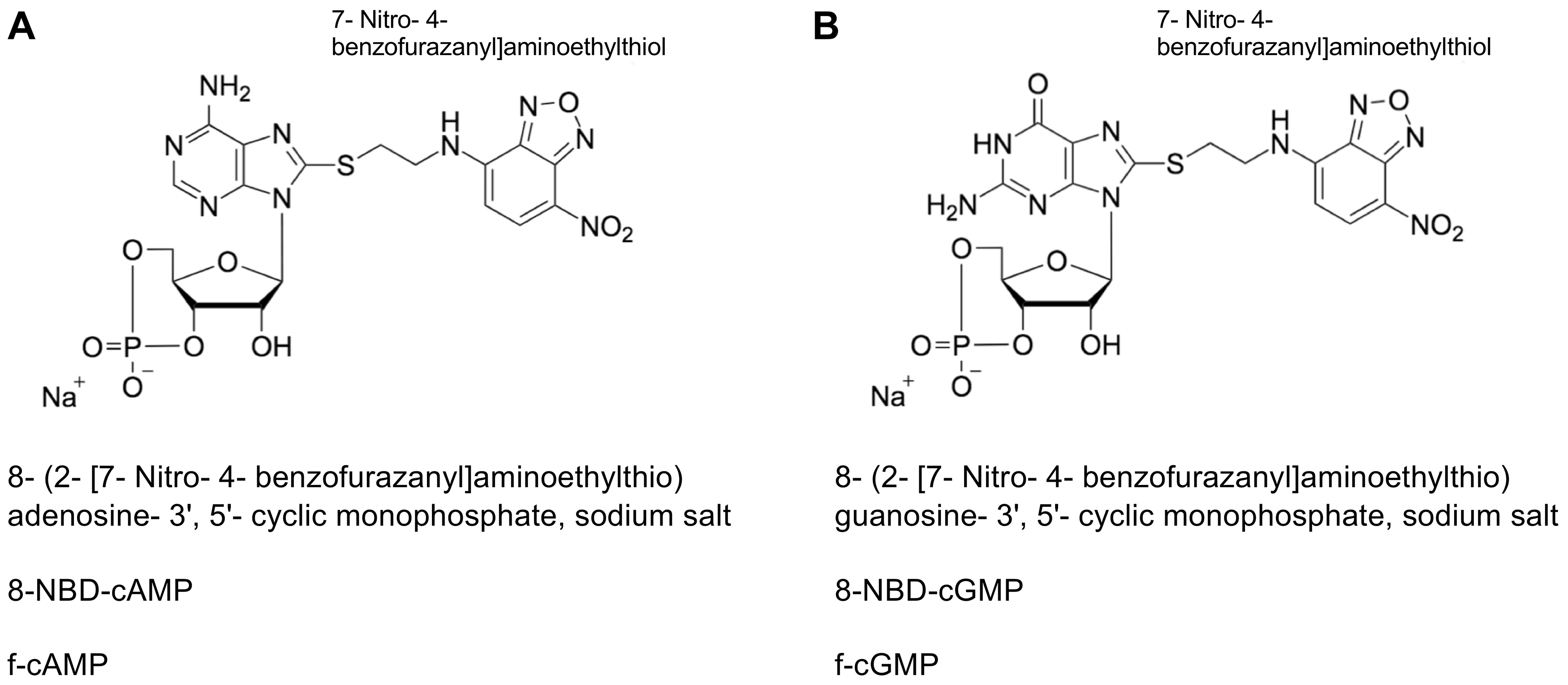
Figure 1. Structural formula of fluorescently labeled cyclic nucleotides. A. 8-NBD-cAMP. B. 8-NBD-cGMP. For both compounds the fluorescent group is labelled separately, the exact chemical name, the abbreviation and the trivial name are given. The representations were adapted from Biolog (Bremen, Germany, www.biolog.de).
Materials and Reagents
- Amicon Ultra-15 Centrifugal Filter MWCO 100 kDa (Merck, catalog number: UFC910024 )
- Amicon Ultra-4 Centrifugal Filter MWCO 100 kDa (Merck, catalog number: UFC810024 )
- BenchMarkTM Pre-stained Protein Ladder (Thermo Fisher Scientific, InvitrogenTM, catalog number: 10748010 )
- Detergent Removal Column (Thermo Fisher Scientific, PierceTM, catalog number: 87779 )
- Ethanol ≥ 95% (Decon Labs, catalog number: V1116 )
- Amphipol A8-35 (Anatrace, catalog number: A835 )
- 3',5'-cyclic AMP, cAMP (Alfa Aesar, catalog number: J62174-03 )
- 3',5'-cyclic GMP, cGMP (Sigma-Aldrich, catalog number: G6129 )
- 8-NBD-cAMP, f-cAMP (BIOLOG, catalog number: N 002 )
- 8-NBD-cGMP, f-cGMP (BIOLOG, catalog number: N 001 )
- Glutaraldehyde (Sigma-Aldrich, catalog number: G6257 )
- HEPES (Simga-Aldrich, catalog number: H3375 )
- KCl (Simga-Aldrich, catalog number: P9333 )
- Superdex 200 10/300 GL (GE Healthcare, catalog number: 17517501 )
- Amphipol stock solution (see Recipes)
- 200 mM cAMP stock solution (see Recipes)
- Assay buffer (see Recipes)
Equipment
- Stir bar (suitable for the cuvette)
- Set of single channel pipettes, ranging from 0.1-1,000 μl (VWR, catalog number: 75788-460 )
- Äkta Chromatography System (GE Healthcare)
- NanoDropTM (Thermo Fisher Scientific)
- Fluorescence-spectrophotometer (HORIBA, PTI QuantaMasterTM, model: 800 )
- UV cuvette (suitable for wavelengths between 230 and 800 nm, volume according to the specifications of the spectrometer between 1 and 4 ml)
- Refrigerated Microcentrifuge (Eppendorf, model: 5418 R )
- Rotator (Fixed Speed Rotator, Cole-Parmer, Stuart, model: SB2 )
Software
- Data can be analyzed using any software capable of programmable, non-linear least-squares fitting (We analyzed the data using GraFit 5)
- GraFit 5.0.13 (Erithacus Software Limited, http://www.erithacus.com/grafit/)
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Schmidpeter, P. A. and Nimigean, C. M. (2018). Fluorescence Titrations to Determine the Binding Affinity of Cyclic Nucleotides to SthK Ion Channels. Bio-protocol 8(19): e3041. DOI: 10.21769/BioProtoc.3041.
分类
分子生物学 > 蛋白质 > 离子通道信号转导
生物化学 > 蛋白质 > 相互作用 > 蛋白质-配体相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link