- EN - English
- CN - 中文
Detection of Cell Death in Planarians
真涡虫细胞死亡的检测
发布: 2018年10月05日第8卷第19期 DOI: 10.21769/BioProtoc.3039 浏览次数: 7778
评审: Ivan ZanoniYang FuAchille Broggi

相关实验方案
利用Cyto-ID®染色和Cytation成像技术定量分析人类成纤维细胞中的自噬小体
Barbara Hochecker [...] Jörg Bergemann
2024年07月05日 1654 阅读
Abstract
Planarians are freshwater flatworms, well known for their ability to regenerate a complete organism from any piece of their body. Furthermore, planarians are constantly growing and degrowing throughout their lives, maintaining a functional and proportioned body. These properties rely on the presence of a population of adult stem cells and on the tight control of their cell renewal, which is based on the balance between the proliferation of new cells and their differentiation, and the death of unnecessary cells. Due to the importance of these two processes in planarian biology, over the years, researchers have optimized molecular techniques to detect both cell proliferation and cell death in planarians. Here, we present the two main protocols currently used for cell death detection and quantification in the planarian field: Caspase-3 activity quantification and TUNEL assay.
Keywords: Caspase-3 activity (Caspase-3活性)Background
Cell renewal in adult organisms is a complex mechanism based on three processes: (a) the elimination of selected cells by cell death; (b) the replacement of eliminated cells through cell division, typically involving adult stem cells and their descendants; and (c) the differentiation of newly generated cells and their integration with preexisting tissue (Pellettieri and Sanchez Alvarado, 2007; González-Estévez and Saló, 2010). In planarians, cell renewal must be continuously coordinated, since they grow and degrow depending on food availability and temperature (Baguñá and Romero, 1981). It is known that the changes in size result mainly from changes in cell number, rather than in cell size, so the ratio of dying/proliferating cells is controlled by environmental conditions (González-Estévez and Saló, 2010). Planarians are able to tolerate long starvation periods, and during this time, they degrow up to minimum sizes. Under these stressful conditions, food reserves from gastrodermis and mesenchyma are the firsts to be used, and at more extreme points, the sexual strains digest the sexual organs, and become asexual (González-Estévez and Saló, 2010; Miller and Newmark, 2012). When food is available, planarians are able to grow back, and in the sexual strains, the reproductive organs reappear. These cycles of grow and degrow occur throughout planarian lives without damage to the animal.
During planarian starvation, cell death increases to re-organize the organs and structures, and planarian adult stem cells (neoblasts) self-renewal is maintained at basal levels, resulting in a decrease of planarian body size (Figures 1A and 1B) (González-Estévez et al., 2012). The tissue remodeling is critical during planarian starvation because it maintains a proportioned planarian body. It was shown that JNK signaling, and Gtdap-1 are controlling the planarian body re-scaling during degrowing through the modulation of apoptotic cell death (González-Estévez et al., 2007; Almuedo-Castillo et al., 2014).
Because cell death is a relevant process in planarian, in the last few years molecular techniques to detect and measure cell death have been developed and optimized. Here, we will explain step-by-step the two main protocols used to detect cell death in planarians: measurement of Caspase-3 activity and TUNEL assay.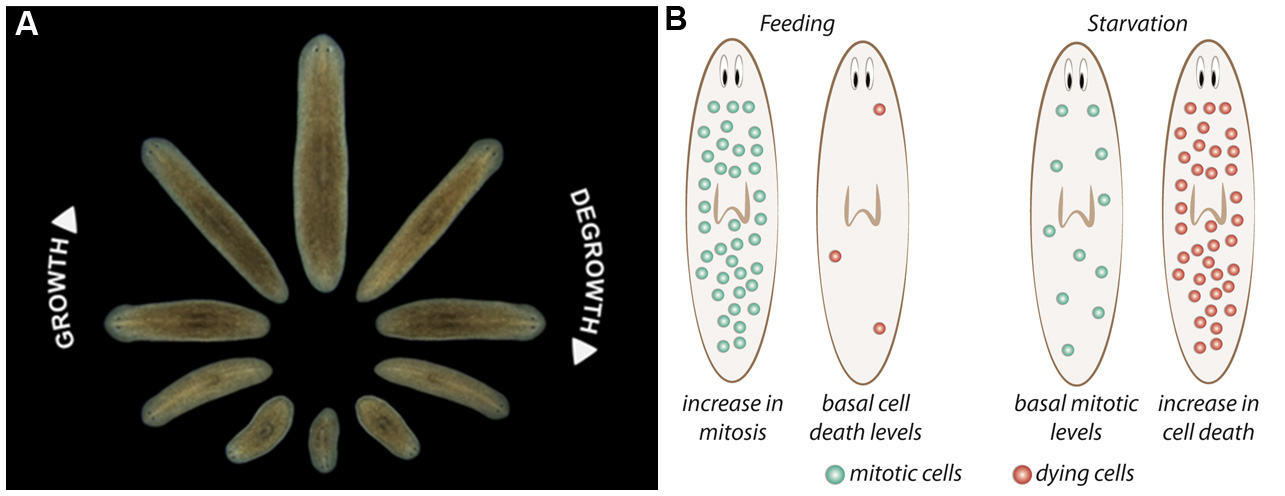
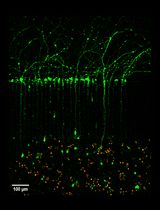
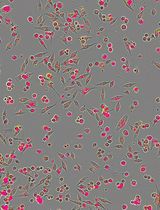
Figure 1. Planarian homeostasis. A. Planarians are able to grow and degrow during their lives, maintaining their body proportions and functionality. Image provided by Gustavo Rodriguez-Esteban. B. After a stimulus, proliferation and/or cell death can change in planarians. After feeding, neoblast proliferation increases throughout planarian body, and cell death is reduced to minimum levels, resulting in the increase of animal’s size. Conversely, when planarians are in starvation, neoblast proliferation is maintained at basal levels and cell death increases, which not only results in a decrease in body size but allows the reorganization of the tissues. Image from Nídia de Sousa Ph.D. thesis (de Sousa, 2017).
Part I: Caspase-3 activity assay
The Caspase-3 activity assay is a fluorescent assay that detects the activity of Caspase-3 in cell lysates using the fluorogenic substrate acetyl Asp-Glu-Val-Asp 7-amido-4-methylcoumarin (Ac-DEVD-AMC). It is based on the hydrolysis of Ac-DEVD-AMC by Caspase-3, resulting in the release of the fluorescent 7-amino-4-methylcoumarin (AMC). AMC that can be detected using a luminescence spectrophotometer with excitation at 380 nm and emission between 420 nm and 460 nm. Cleavage of the substrate only occurs in lysates in which Caspase-3 is present, which is a gene required for apoptosis; therefore, the amount of AMC produced is proportional to the number of apoptotic cells in the sample.
Materials and Reagents
- Petri dish (VWR, catalog number: 391-0439 )
- Slides (VWR, catalog number: 631-1551 )
- Razor blade (MARTOR, catalog number: NO. 743 )
- Eppendorf tubes (VWR, Eppendorf, catalog number: 700-5239 )
- 15 ml Falcons (LF Equipamentos, catalog number: 166 )
- Spectrophotometry Cuvettes (VWR, catalog number: 634-0677BTU )
- 96-well plate (VWR, Corning, catalog number: 734-1664 )
- MilliQ water
- Ice
- Micro BCA Protein Assay Kit (Thermo Fisher Scientific, PierceTM, catalog number: 23235 )
- Tris-HCl, pH 8 (Sigma-Aldrich, catalog number: 93362 )
- EDTA, pH 8 (Sigma-Aldrich, catalog number: 1233508 )
- Triton X-100 (Sigma-Aldrich, catalog number: X100 )
- HEPES pH 7.5 (Sigma-Aldrich, catalog number: H3375 )
- Glycerol 10% (Sigma-Aldrich, catalog number: G5516 )
- DTT (Sigma-Aldrich, catalog number: 646563 )
- Caspase-3 inhibitor Z-DEVD-FMK (Merck, catalog number: 264155 )
- Caspase-3 substrate Ac-DEVD-AMC (BD Biosciences, PharmingenTM, catalog number: 556449 )
- Lysis buffer (see Recipes)
- Assay buffer (see Recipes)
Equipment
- Oven
- Luminescence spectrophotometer
- Pipettes
- Vortex
- Centrifuge
- Platform shaker
- 4 °C refrigerator
- -20 °C freezer
- 37 °C incubator
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Sousa, N. D. and Adell, T. (2018). Detection of Cell Death in Planarians. Bio-protocol 8(19): e3039. DOI: 10.21769/BioProtoc.3039.
分类
发育生物学 > 细胞生长和命运决定 > 增殖
癌症生物学 > 细胞死亡 > 动物模型
细胞生物学 > 细胞活力 > 细胞死亡
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link