- EN - English
- CN - 中文
Soluble and Solid Iron Reduction Assays with Desulfitobacterium hafniense
哈夫尼脱亚硫酸杆菌用于可溶和固体铁的还原试验
发布: 2018年09月05日第8卷第17期 DOI: 10.21769/BioProtoc.3002 浏览次数: 6639
评审: Valentine V TrotterKarolina SubrtovaAnonymous reviewer(s)
Abstract
There is a pressing need to develop sustainable and efficient methods to protect and stabilize iron objects. To develop a conservation-restoration method for corroded iron objects, this bio-protocol presents the steps to investigate reductive dissolution of ferric iron and biogenic production of stabilizing ferrous iron minerals in the strict anaerobe Desulfitobacterium hafniense (strains TCE1 and LBE). We investigated iron reduction using three different Fe(III) sources: Fe(III)-citrate (a soluble phase), akaganeite (solid iron phase), and corroded coupons. This protocol describes a method that combines spectrophotometric quantification of the complex Fe(II)-Ferrozine® with mineral characterization by scanning electron microscopy and Raman spectroscopy. These three methods allow assessing reductive dissolution of ferric iron and biogenic mineral production as a promising alternative for the development of an innovative sustainable method for the stabilization of corroded iron.
Keywords: Reductive dissolution of ferric iron (铁离子的还原性溶解作用)Background
Since the Iron Age, iron has been used to produce everyday utensils. Therefore, archaeological iron findings are an extremely important testimony of the past and should be preserved. However, due to its reactivity, iron can be easily corroded and archaeological iron objects risk to be completely damaged. When buried, iron artifacts develop a complex corrosion layer according to the environmental conditions of the burial site. After excavation, conditions change and the corrosion layer becomes unstable. To avoid complete destruction, archaeological iron objects require a rapid stabilization treatment. Currently, available stabilization treatments do not provide long-term protection and have substantial drawbacks, such as toxicity, low efficiency, and production of large amount of waste (Scott and Eggert, 2009; Rimmer et al., 2012). Consequently, it is necessary to develop new technologies to stabilize archaeological iron artifacts.
Exploiting a microbial metabolism is increasingly considered for the development of more efficient, sustainable and eco-friendly treatments in conservation-restoration (Ranalli et al., 2005; Cappitelli et al., 2006 and 2007; Jonkers, 2011; Joseph et al., 2011, 2012 and 2013; Bosch-Roig and Ranalli, 2014). Our research team is developing a treatment based on the reductive dissolution of ferric iron under anaerobic conditions (Kooli et al., 2018; Comensoli et al., 2017). The unstable corrosion products are converted into more stable biogenic minerals (i.e., magnetite and vivianite), as a byproduct of bacterial iron reduction. This conversion would stabilize the corrosion layer of the object.
In order to study the suitability of the chosen bacteria, iron reduction has to be carefully monitored. Several methods are available to quantify iron. Inductive coupled plasma mass spectrometry (ICP-MS) is useful to measure trace elements with concentrations of less than 1 ppm (Meissner et al., 2004). However, it requires expensive equipment and does not provide information on the oxidation state of iron if not combined with chromatographic separation devices such as high-performance liquid chromatography (HPLC), ion chromatography (IC), gas chromatography (GC), and capillary electrophoresis (CE) (Thomas, 2013). A spectrophotometric method to measure Fe(II) uses the metal-ligand ortho-phenanthroline (Fortune and Mellon, 1938). This compound is now considered carcinogenic (Whittaker et al., 2001). Therefore, for this protocol we selected the spectrophotometric quantification of Fe(II) with the Ferrozine® assay. This simple and reliable method requires standard lab equipment and can be used to analyze many samples. In addition, the characterization of biogenic minerals was made based on their appearance, morphology and molecular composition. For these analyses, we used scanning electron microscopy and Raman spectroscopy.
This Bio-protocol consists of three main steps (Figure 1): A. Biomass production; B. Incubation with iron sources; C. Validation of iron reduction.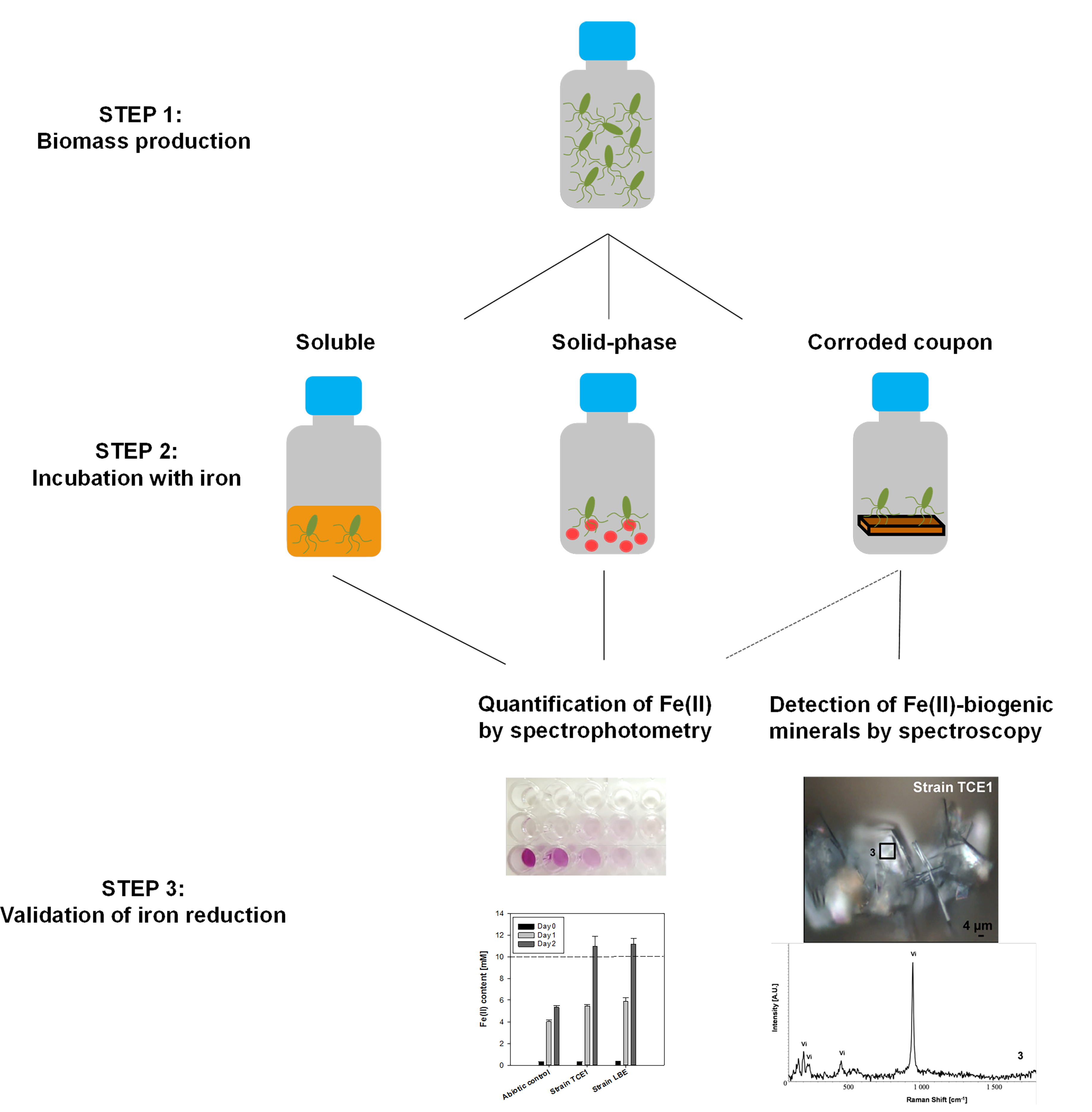
Figure 1. Graphical summary of the overall structure of this bio-protocol
Materials and Reagents
- 1.7 ml Eppendorf centrifuge tubes (Corning, Axygen®, catalog number: MCT-175-C )
- Syringes
1 ml (CODAN, catalog number: 621640 )
5 ml (CODAN, catalog number: 625607 )
20 ml (CODAN, catalog number: 627602 ) - Needle for syringes (Henke-Sass, Wolf, catalog number: 4710005016 )
- 1,000, 500, 100 and 50 ml serum bottle for anaerobic bacterial culture (DWK Life Sciences, Wheaton, catalog number: W012467A [100 ml])
- 100 ml serum bottle with large bottleneck (Merck, catalog number: STBMRFA12 )
- Rubber stoppers for serum bottles (VWR, special request)
- Metal caps for serum bottle (Thermo Fisher Scientific, catalog number: C4020-3A )
- Serum bottle seal crimper (DWK Life Sciences, Wheaton, catalog number: 224322 )
- 0.2 μm sterile filter (SARSTEDT, catalog number: 83.1826.001 )
- 96-well polypropylene microplate (SARSTEDT, catalog number: 82.1581 )
- 96-well microcentrifuge tube flipper rack with Lid (Fisher Scientific, catalog number: 11710344 )
- Desulfitobacterium hafniense strain TCE1 (Gerritse et al., 1999)
- Desulfitobacterium hafniense strain LBE (Comensoli et al., 2017)
- Ethanol (Thommen Furler, catalog number: 180-VL54K )
- Corroded iron coupons (steel coupons presenting a natural corrosion layer produced after outdoor exposure in the city of Zurich, Switzerland)
- Adhesive Carbon Tape 12 mm x 20 m (Agar Scientific, catalog number: AGG3939A )
- N2 gas cylinder (Carbagas, catalog number: I4001 )
- NH4HCO3 (Sigma-Aldrich, catalog number: A6141 )
- NaHCO3 (Sigma-Aldrich, catalog number: S5761 )
- K2HPO4•3H2O (Sigma-Aldrich, catalog number: P5504 )
- NaH2PO4•2H2O (Sigma-Aldrich, catalog number: 71505 )
- Peptone (BD, catalog number: 211677 )
- Resazurin sodium salt (Sigma-Aldrich, catalog number: R7017 )
- Cyanocobalamin (Acros Organics, catalog number: 405920010 )
- Riboflavin (Sigma-Aldrich, catalog number: R4500 )
- Thiamine-hydrochloride (AppliChem, catalog number: A0955 )
- Biotin (Thermo Fisher Scientific, Alfa Aesar, catalog number: A14207 )
- P-aminobenzoate (sodium salt) (Sigma-Aldrich, catalog number: A9878 )
- Pantothenate (sodium salt) (Sigma-Aldrich, catalog number: P3161 )
- Folic acid•2H2O (Sigma-Aldrich, catalog number: F7876 )
- Lipoic acid (Sigma-Aldrich, Fluka, catalog number: 62320 )
- Pyridoxine hydrochloride (Acros Organics, catalog number: 150770500 )
- Nicotinic acid (Sigma-Aldrich, catalog number: N4126 )
- EDTA disodium salt•2H2O (Sigma-Aldrich, catalog number: E1644 )
- FeCl2•4H2O (Sigma-Aldrich, catalog number: 44939 )
- MnCl2•4H2O (Sigma-Aldrich, catalog number: M3634 )
- CoCl2•6H2O (Sigma-Aldrich, catalog number: C8661 )
- ZnCl2 (Sigma-Aldrich, catalog number: 793523 )
- CuCl2•2H2O (Sigma-Aldrich, catalog number: C3279 )
- AlCl3 (Sigma-Aldrich, catalog number: 237051 )
- H3BO3 (Sigma-Aldrich, catalog number: B6768 )
- Na2MoO4•2H2O (Sigma-Aldrich, catalog number: 331058 )
- NiCl2•6H2O (Sigma-Aldrich, catalog number: N6136 )
- CaCl2•2H2O (Sigma-Aldrich, catalog number: 223506 )
- MgCl2•6H2O (Sigma-Aldrich, catalog number: M2393 )
- Na2S•9H2O (Sigma-Aldrich, catalog number: 208043 )
- Sodium DL-lactate 60% solution (Sigma-Aldrich, catalog number: L1375 )
- Disodium fumarate (Sigma-Aldrich, catalog number: F1506 )
- HCl 37% (S-20) (Honeywell International, catalog number: 30721-1L-GL )
- MilliQ water
- Fe(II)-ammonium sulfate (Honeywell International, Fluka, catalog number: 09720 )
- Fe(III)-citrate (Sigma-Aldrich, Fluka, catalog number: 44941-250G )
- 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) (HEPES) (Sigma-Aldrich, catalog number: H3375-250G )
- NaOH (Sigma-Aldrich, catalog number: 71690 )
- Goethite: α-FeO(OH) (Sigma-Aldrich, catalog number: 71063-100G ) (alternative source of solid Fe(III)-phase to akaganeite)
- Fe2O3 (Sigma-Aldrich, catalog number: 529311-5G ) (alternative source of solid Fe(III)-phase to akaganeite)
- Growth medium for D. hafniense (see Recipes)
- N2-degassed H2O
- Sterile serum bottles
- Solution of sodium DL-lactate 40% (v/v)
- Solution of disodium fumarate 16% (v/v)
- Reducing agent solution 1 M
- Resazurin solution 0.5 g/L
- Vitamin solution 1
- Vitamin solution 2
- Vitamin solution 3
- Vitamin solution 4
- Trace elements solution
- Carbonate solution
- Solution A (basal medium)
- Solution B (vitamin solution)
- Solution C (buffering/reducing solution)
- Solution D
- Soluble Fe(III)-citrate (35 g/L) – 100 ml (see Recipes)
- HCl solutions to adjust pH
- NaOH solutions to adjust pH
- Fe(III) solution
- Solid Fe(III) suspension (see Recipes)
- Solid Fe(III) source
- Preparation of the suspension of solid Fe(III)-phase (akaganeite or goethite)
- Ferrozine® reagents (see Recipes)
- HCl solution 5 M
- Stock solution of Fe(II) 1 M for calibration curve
- Ferrozine® reagent
Equipment
- 1 L graduated flasks (SciLabware, catalog number: 1132/26 )
- Magnetic bars (Sigma-Aldrich, BRAND, catalog numbers: Z328774 , Z328812 )
- Stainless steel spatula (Sigma-Aldrich, catalog number: HS15909 )
- Balance (Mettler-Toledo International, catalog number: PG5002 )
- P20 pipetman (Gilson, catalog number: F123600 )
- P200 pipetman (Gilson, catalog number: F123601 )
- P1000 pipetman (Gilson, catalog number: F123602 )
- pH meter
- Bunsen burner (FIREBOY Plus) (Integra Biosciences, catalog number: 144000 )
- Autoclave (Fedegari Autoklav FOB5/TS) (VITARIS, catalog number: 260000-FED , serial number: NBD801AV)
- Orbital shaker (Kühner, model: SMX1200 )
- Hotplate and magnetic Stirrer (Heidolph Instruments, catalog number: MR2002 )
- Spinbar® Magnetic Stir bar (Sigma-Aldrich, SP Scienceware - Bel-Art Products - H-B Instrument, catalog number: Z126942-1EA )
- Spectrophotometer cuvettes (Sigma-Aldrich, catalog number: C5291-100EA )
- Spectrophotometer UV-visible (GENESYSTM 10S) (Thermo Fisher Scientific, catalog number: 840-208100 )
- Microplate reader (Biochrom, Asys Hitech, catalog number: UVM 340 )
- pH meter (Benchtop Meter AE150) (Fisher Scientific, catalog number: 15524693 )
- Biosafety cabinet equipped with UV lamp at 254 nm (Azbil Telstar, catalog number: Bio II Advance )
- Chemical fume hood
- Desiccator (BRAND, catalog number: 65815 )
- Scanning electron microscope (SEM) (Philips ESEM XL30 FEG environmental scanning electron microscope equipped with an energy-dispersive X-ray analyzer (Philips)
- Raman Microscope (HORIBA, JOBIN YVON, LabRAM Aramis microscope equipped with a Nd:YAG laser of 532 nm and controlled by LabSpec NGS spectral software. HORIBA, JOBIN YVON, catalog number: LabRAM Aramis, 3 lasers and xyz stage)
- Vortex
- Vacuum pump
- Fridge
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Comensoli, L., Maillard, J., Kooli, W. M., Junier, P. and Joseph, E. (2018). Soluble and Solid Iron Reduction Assays with Desulfitobacterium hafniense. Bio-protocol 8(17): e3002. DOI: 10.21769/BioProtoc.3002.
分类
微生物学 > 微生物新陈代谢 > 其它化合物
生物化学 > 其它化合物 > 离子 > 铁
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link