- EN - English
- CN - 中文
Measuring CD38 Hydrolase and Cyclase Activities: 1,N6-Ethenonicotinamide Adenine Dinucleotide (ε-NAD) and Nicotinamide Guanine Dinucleotide (NGD) Fluorescence-based Methods
基于荧光的测量CD38水解酶和环化酶活性的方法:以1,N6-乙烯基烟酰胺腺嘌呤二核苷酸(ε-NAD)和烟酰胺鸟嘌呤二核苷酸(NGD)为底物
(*contributed equally to this work) 发布: 2018年07月20日第8卷第14期 DOI: 10.21769/BioProtoc.2938 浏览次数: 9353
评审: Andrea PuharYONG TENGAnca Savulescu
Abstract
CD38 is a multifunctional enzyme involved in calcium signaling and Nicotinamide Adenine Dinucleotide (NAD+) metabolism. Through its major activity, the hydrolysis of NAD+, CD38 helps maintain the appropriate levels of this molecule for all NAD+-dependent metabolic processes to occur. Due to current advances and studies relating NAD+ decline and the development of multiple age-related conditions and diseases, CD38 gained importance in both basic science and clinical settings. The discovery and development of strategies to modulate its function and, possibly, treat diseases and improve health span put CD38 under the spotlights. Therefore, a consistent and reliable method to measure its activity and explore its use in medicine is required. We describe here the methods how our group measures both the hydrolase and cyclase activity of CD38, utilizing a fluorescence-based enzymatic assay performed in a plate reader using 1,N6-Ethenonicotinamide Adenine Dinucleotide (ε-NAD) and Nicotinamide Guanine Dinucleotide (NGD) as substrates, respectively.
Keywords: CD38 (CD38)Background
Current studies on age-related development of metabolic dysfunction and frailty are each day in more evidence. It is known that, as the aging progresses, the NAD+ levels decrease in an expected process. Recent studies have shown that a reduction in nicotinamide adenine dinucleotide (NAD+) is implicated in the development of age-associated metabolic decline (Massudi et al., 2012). Increased NAD+ levels in vivo, results in activation of pro-longevity and health span-related factors and improves several physiological and metabolic parameters of aging (Camacho-Pereira et al., 2016), including muscle function, exercise capacity, glucose tolerance, and cardiac function in mouse models of natural and accelerated aging.
Due to its role in NAD+ metabolism, the study of CD38 and its functions has been of great importance. CD38 was first identified in 1980 as a structural cell surface marker for the characterization of immune cells (Malavasi et al., 2008; van de Donk et al., 2016), and its first association as a NAD hydrolase enzyme was in the following decade (Kontani et al., 1993). However, during the past years, its enzymatic activities were more clearly elucidated. Initially, CD38 has been implicated to be responsible for the synthesis of the second messengers, cyclic ADP-ribose (cADPR), ADPR and nicotinic acid–adenine dinucleotide phosphate (NAADP) (Chini et al., 2002). These products are involved in calcium signaling and control many biological processes including lymphocyte proliferation and insulin secretion (Kato et al., 1999). However, its major enzymatic activity is the NAD+ hydrolysis, placing CD38 as the major NADase in several mammalian tissues and as an important regulator of NAD+-dependent processes (Aksoy et al., 2006).
The primary catalytic reaction of CD38 involves the cleavage of the high energy β-glycosidic bond between nicotinamide and ribose. During catalysis, the removal of the nicotinamide from β-NAD is coupled with the formation of intermediates that are stabilized through H-bonds between their ribosyl groups and the catalytic residue Glu226, a residue required for the NADase and cyclase activity of the enzyme (Sauve et al., 2000; Liu et al., 2009). These intermediates are released from the catalytic site forming ADPR or cADPR (Figure 1). In general, the majority of the CD38 NADase catalytic activity will generate nicotinamide, but also ADPR and cADPR which have been shown to have second messenger signaling roles through the activation of ryanodine receptor (RYR2). The full description of these pathways and Ca2+ signaling can be found in the remarkable works of Galione (1994) and Chini and Dousa (1996). The roles of CD38 as a cyclase and of NAD-derived calcium messengers in physiology and pathology have been extensively reviewed (Sauve et al., 2000; Chini, 2009).
Physiologically, CD38 has been implicated in the regulation of metabolism and the pathogenesis of the aging process, and of multiple conditions, such as obesity, diabetes, heart disease, asthma and inflammation. Therefore, the study of CD38, its activities, and possible modulators are of great interest. Our protocol presents a method of measuring its hydrolase and cyclase activities, utilizing ε-NAD and NGD techniques (Graeff et al., 1994) with a fluorescence-based enzymatic assay performed in a plate reader, in a consistent and reproducible manner (Figure 2).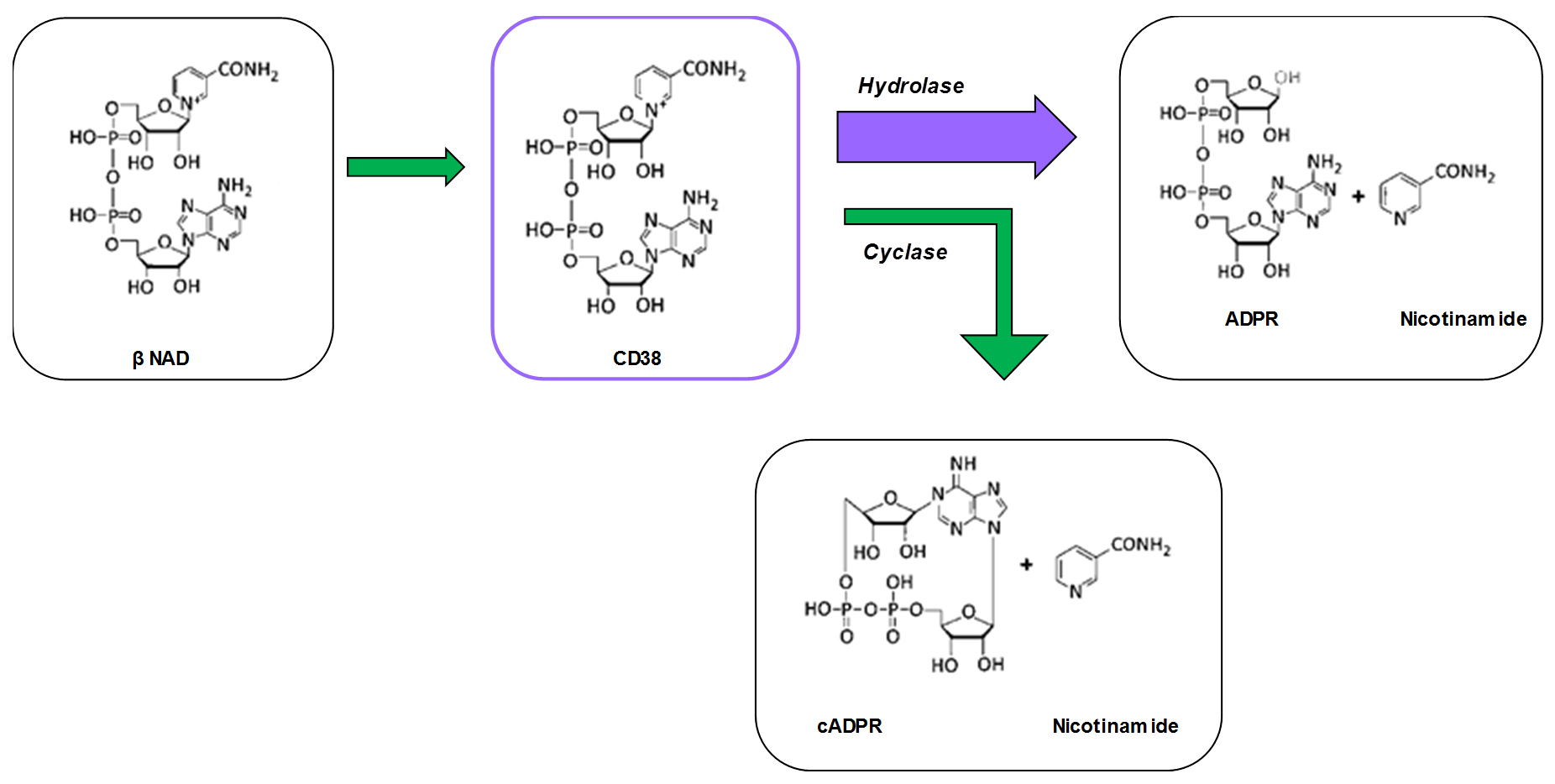
Figure 1. Schematic illustrating the reactions catalyzed by CD38 under physiological conditions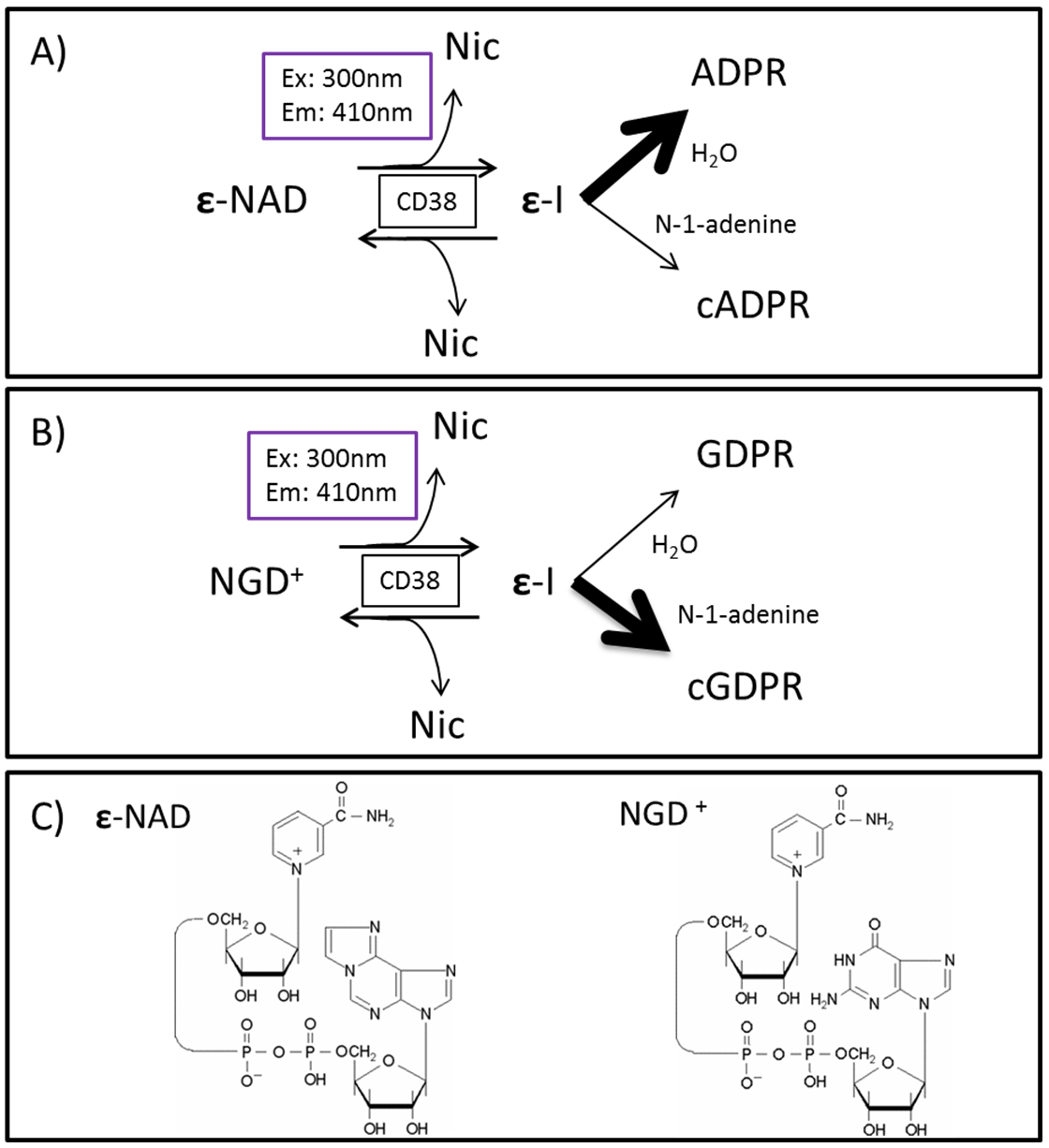
Figure 2. CD38 and substrate schematics. A. CD38 hydrolase activity (ε-NAD as substrate); B. Cyclase activity (NGD as substrate); Strong arrow indicates which product is preferentially formed in each reaction. C. Molecular structure of ε-NAD and NGD. *Nic = Nicotinamide, ε-I = enzyme-intermediate complex, (c)ADPR = (cyclic) ADP-ribose, (c)GDPR = (cyclic) GDP-ribose, Ex = excitation wavelength, Em = emission wavelength.
Materials and Reagents
- Plastic tips 1,000 (Thermo Scientific®, Molecular Bioproducts, catalog number: 3101 )
- Plastic tips 200 (Thermo Fisher Scientific, Molecular Bioproducts, catalog number: 3551 )
- 96-well plate (Microfluor 1 White flat-bottom plate) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 7705 )
- 1.5 ml tubes
- 60 mm culture dishes (Fisher Scientific, FisherbrandTM, catalog number: FB012921 )
- Tissues of interest: any tissue can be used to measure NAD+/NADH levels
- Cells of interest: we usually use A549, JURKAT, Patu 9888T to measure NAD+/NADH levels
- Bovine Serum Albumin (BSA) (Sigma-Aldrich, catalog number: A7906 )
- Sucrose (Sigma-Aldrich, catalog number: S0389-1KG )
- Tris Base (Trizma® base, Sigma-Aldrich, catalog number: T6066 )
- Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories, catalog number: 5000006 )
- CD38 human recombinant enzyme (R&D Systems, catalog number: 2404-AC-010 )
- CD38 inhibitor (Merck, Calbiochem, catalog number: 538763 )
- Anti-CD38 antibody–Isatuximab (Creative-Biolabs, catalog number: TAB-432CQ )
- Nicotinamide guanine dinucleotide sodium salt (NGD) (Sigma-Aldrich, catalog number: N5131 )
- Nicotinamide 1, N6-ethenoadenine dinucleotide (ε-NAD) (Santa Cruz Biotechnology, catalog number: sc-215559 )
- MES (Sigma-Aldrich, catalog number: M3671 )
- Sodium chloride (Sigma-Aldrich, catalog number: S7653 )
- Nanopure water
- HCl
- Sucrose Buffer (see Recipes)
- rhCD38 enzyme buffer (see Recipes)
Materials necessary to collect cells:
- Scraper–Corning cell lifter (Corning, catalog number: 3008 )
- Trypsin-EDTA 0.25% (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056 )
- Phosphate Buffered Saline (PBS) 1x (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023 )
Equipment
Note: The brands and models indicated are the ones used by our group, similar equipment can be used as well.
- Pipettes (10, 200, 1,000 μl)
- Scissors
- Graduated cylinder
- Repeat pipette (Eppendorf, model: Repeater® M4 )
- Scale (Mettler-Toledo International, model: AG104 )
- Microcentrifuge (Eppendorf, model: 5424 )
- Homogenizer (Tissue Tearor, Bio Spec Products, catalog number: 780CL-04 )
- Sonic Dismembrator (Fisher Scientific, model: Model 100 Sonic Dismembrator)
- Spectrophotometer (BioTek Instruments, model: Epoch 2 )
- Vortex (Scientific Industries, model: Vortex-Genie 2 , catalog number: G560)
- Plate reader (Molecular Devices, model: SpectraMax Gemini XPS )
Software
- Gen5 Microplate Reader and Imager Software (BioTek Instruments)
- Microsoft Excel (Microsoft Corporation)
- SoftMax Pro 6 (Molecular Devices, LLC)
- GraphPad Prism 7 (GraphPad Software, Inc)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
de Oliveira, G. C., Kanamori, K. S., Auxiliadora-Martins, M., Chini, C. C. S. and Chini, E. N. (2018). Measuring CD38 Hydrolase and Cyclase Activities: 1,N6-Ethenonicotinamide Adenine Dinucleotide (ε-NAD) and Nicotinamide Guanine Dinucleotide (NGD) Fluorescence-based Methods. Bio-protocol 8(14): e2938. DOI: 10.21769/BioProtoc.2938.
分类
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link