- EN - English
- CN - 中文
Detection of Catalase Activity by Polyacrylamide Gel Electrophoresis (PAGE) in Cell Extracts from Pseudomonas aeruginosa
聚丙烯酰胺凝胶电泳(PAGE)检测铜绿假单胞菌细胞提取物中的过氧化氢酶活性
发布: 2018年06月05日第8卷第11期 DOI: 10.21769/BioProtoc.2869 浏览次数: 9369
评审: Dennis NürnbergMichael EnosDeena Jacob
Abstract
Bacteria in nature and as pathogens commonly face oxidative stress which causes damage to proteins, lipids and DNA. This damage is produced by the action of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), singlet oxygen, superoxide anion and hydroxyl radical. ROS are generated by antimicrobials, environmental factors (e.g., ultraviolet radiation, osmotic stress), aerobic respiration, and host phagocytes during infective processes. Pseudomonas aeruginosa, a versatile bacterium, is a prevalent opportunistic human pathogen which possesses several defense strategies against ROS. Among them, two catalases (KatA and KatB) have been well characterized by their role on the defense against multiple types of stress. In this protocol, KatA and KatB activities are detected by polyacrylamide gel electrophoresis (PAGE). It is also suggested that the detection of KatB is elusive.
Keywords: Pseudomonas aeruginosa (铜绿假单胞菌)Background
P. aeruginosa is a ubiquitous bacterium that can be found in a free form in terrestrial and aquatics habitats or as an opportunistic human pathogen causing fatal infections in immunocompromised individuals, patients with skin damage or cystic fibrosis. To defend itself from ROS generated by its strong aerobic metabolism, host phagosomal vacuoles and environmental factors, this microorganism possesses several antioxidative strategies. Among them, two monofunctional catalases (KatA and KatB) are responsible for decomposing H2O2 to water and O2. KatA is the main catalase and has unique characteristics: it is unusually stable and essential to H2O2 resistance, osmoprotection and virulence (Hassett et al., 2000; Lee et al., 2005). It has been suggested that the stability of KatA is one of the main factors for the high level activity under normal growth conditions, and for this reason, katA has been regarded as a constitutively expressed gene in P. aeruginosa (Heo et al., 2010). However, it has been reported that KatA activity is induced in the stationary growth phase (up to 10-fold) and by increased levels of H2O2 (Brown et al., 1995; Suh et al., 1999; Heo et al., 2010). Moreover, katA expression has been demonstrated to be modulated by the global regulator OxyR and Quorum Sensing, whose activation depends on increased levels of H2O2 and high cell density, respectively (Hassett et al., 1999; Heo et al., 2010). KatB is only detected in the presence of H2O2 or paraquat and is partially involved in resistance to oxidative stress (Brown et al., 1995; Lee et al., 2005).
Solar ultraviolet-A (UVA) radiation is one of the main environmental stress factors for P. aeruginosa. Given the oxidative nature of UVA damage, we studied the role of catalases in defense of this microorganism against radiation. We demonstrated that KatA is essential in the optimal response against lethal doses of UVA, both in planktonic cells and biofilms (Costa et al., 2010; Pezzoni et al., 2014). In addition, we reported that low doses of UVA increase KatA and KatB activity and that this regulation occurs at the transcriptional level (Pezzoni et al., 2016). This phenomenon is relevant since it constitutes an adaptive mechanism that prevents cell damage by subsequent exposure to lethal doses of UVA, H2O2, or sodium hypochlorite (Pezzoni et al., 2016).
In the course of our studies, it became necessary to do an in-depth analysis of catalase activity. The total catalase activity in cell extracts was quantified by following spectrophotometrically the decomposition of H2O2, according to Aebi (1984). However, this assay cannot distinguish between KatA and KatB activities. To analyze individual catalase activity, we implement the method proposed by Wayne and Díaz (1986). In brief, crude cell extracts are loaded onto non-denaturing polyacrylamide gels (PAGE), and both catalases are separated by their differential electrophoretic motility; colorless bands of catalase activity are revealed by incubation of the gel with H2O2 and subsequent addition of a ferric chloride-potassium ferricyanide solution. The principle of this method involves the reaction of H2O2 with potassium ferricyanide (III) by reducing it to ferrocyanide (II); the peroxide is oxidized to O2. Ferric chloride reacts with ferrocyanide (II) to form an insoluble blue pigment. Because of the action of catalase on H2O2 decomposition, areas where this enzyme is active develop as clear bands in a blue gel (Patnaik et al., 2013). Additional papers were consulted to fine-tune this technique (Brown et al., 1995; Hassett et al., 1999; Elkins et al., 1999). The studies were performed with the prototypical P. aeruginosa strain PAO1 and isogenic derivatives PW8190 (katA::IslacZ/hah) and PW8769 (katB::IslacZ/hah) carrying mutations into katA and katB, respectively. Mutant strains devoid for KatA or KatB are useful to analyze the role of each enzyme in response to stress and as controls in PAGE catalase assays.
In this protocol, we describe how to detect individual catalase activity by PAGE using cell extracts from P. aeruginosa. Because of the particular characteristics of KatA (high abundance and stability), its detection does not present major difficulties. On the contrary, KatB detection is elusive, so that two changes were applied to the conventional technique: a protein extraction reagent was used instead of sonication to prepare the cell extracts, and the electrophoresis was performed at 4 °C. Based on these assays, it was concluded that KatB is an unstable enzyme, a fact that should be taken into account in quantitative or qualitative catalase assays under inducing (oxidative) conditions.
Materials and Reagents
- Pipette tips
- 50 ml sterile conical Falcon tubes (Nunc® EZ FlipTM, Thermo Fisher Scientific, catalog number: 362696 )
- 1.5 ml sterile Eppendorf centrifuge tubes (Eppendorf, catalog number: 022364111 )
- Sonication device
Note: This was assembled in our laboratory by attaching four plastic tubes (3 cm diameter, 3 cm high) to a plastic box (Figure 1).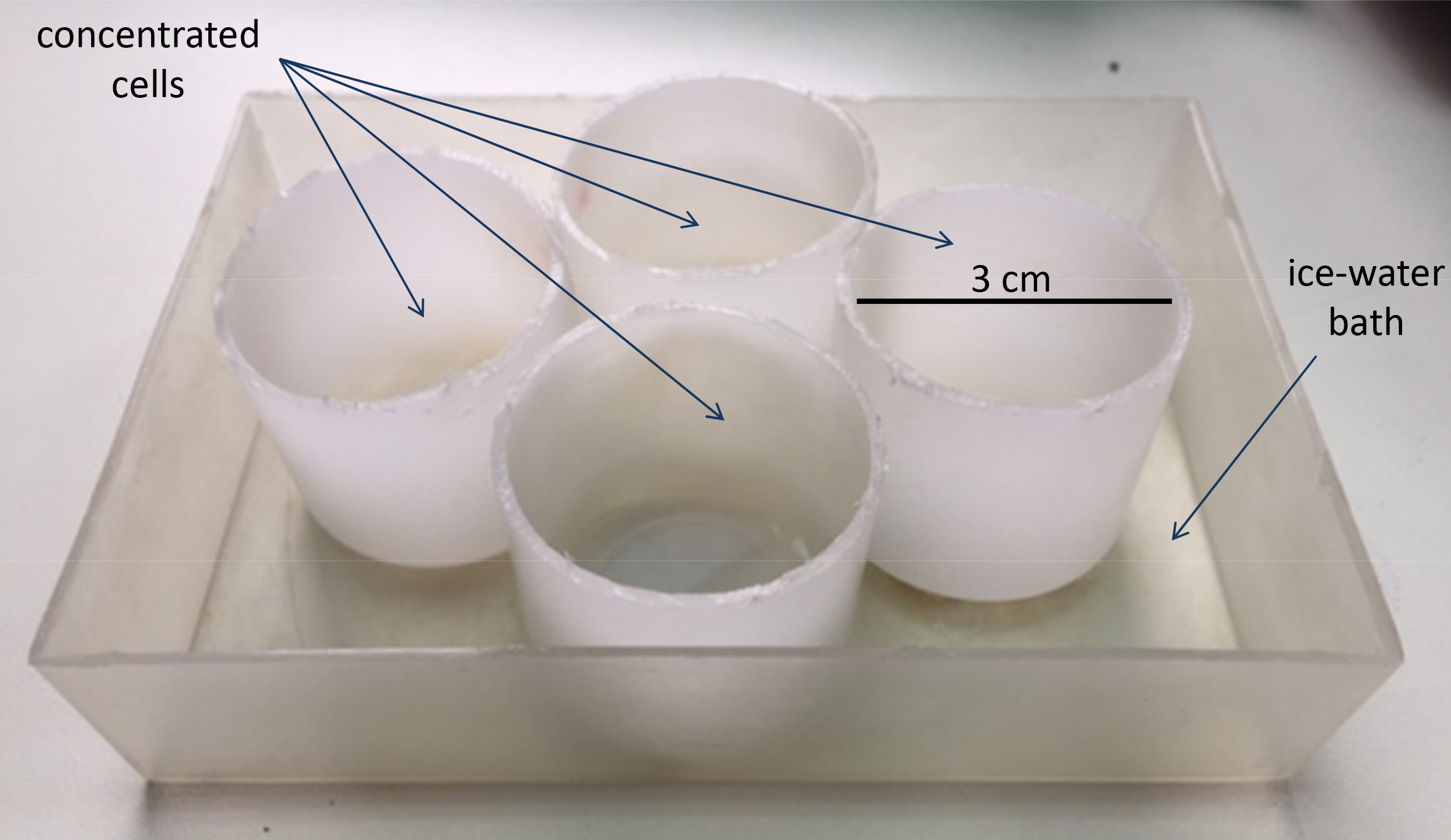
Figure 1. Sonication device - Paper towel (WypAll* X 60 Jumbo Roll, KCWW, Kimberly-Clark, catalog number: 30218593 )
- Spatula
- Pyrex tray (Pyrex® Storage 13 x 18 cm)
- P. aeruginosa strains
Note: PAO1, referred to as the wild-type, and catalase mutants PW8190 and PW8769 were obtained from the Washington Genome Center. Catalase mutants were constructed by insertion of IslacZ/hah transposon into katA (PW8190, hereinafter KatA-less strain) or katB (PW8769, hereinafter KatB-less strain) (Jacobs et al., 2003). - Distilled water
- Tryptone (Oxoid, catalog number: LP0042 )
- Yeast extract (Merck, catalog number: 103753 )
- Sodium chloride (NaCl) (Biopack, catalog number: 1646.08 )
- Albumin from bovine serum (Sigma-Aldrich, catalog number: A4378 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S3264 )
- Sodium phosphate (NaH2PO4) (Sigma-Aldrich, catalog number: S0751 )
- Hydrogen peroxide (H2O2) 30% (Merck, catalog number: 107210 )
- Bugbuster Protein Extraction Reagent (Merck, Novagen, catalog number: 70584-4 )
- Sodium thiosulfate (Na2S2O3) (Avantor Performance Materials, MacronTM, catalog number: 8100-04 )
- Ammonium persulfate ((NH4)2S2O8) (MP Biomedicals, catalog number: 04802811 )
- TEMED (MP Biomedicals, catalog number: 02195516 )
- Trizma base (Tris[hydroxymethyl]aminomethane) (C4H11NO3) (Sigma-Aldrich, catalog number: T1503 )
- Glycine (Sigma-Aldrich, catalog number: G7126 )
- Hydrochloric acid fuming 37% (HCl) (Merck, catalog number: 100317 )
- Acrylamide (Sigma-Aldrich, catalog number: A8887 )
- Bisacrylamide N,N’-methylene-bis-acrylamide (Sigma-Aldrich, catalog number: M7256 )
- EDTA (Merck, Calbiochem, catalog number: 324503 )
- Sodium hydroxide (NaOH) (Avantor Performance Materials, MacronTM, catalog number: 7708 )
- Glycerol (Merck, catalog number: 104094 )
- Bromophenol blue (VWR, DBH, catalog number: 20015 )
- Ferric chloride (FeCl3) (Avantor Performance Materials, MacronTM, catalog number: 5029-04 )
- Potassium ferricyanide (K3Fe (CN)6) (UCB, catalog number: b1599 )
- LB medium (see Recipes)
- 4 M NaCl (see Recipes)
- Saline solution (see Recipes)
- 50 mM sodium phosphate buffer, pH 7 (see Recipes)
- 30 mM H2O2 (see Recipes)
- 4 mM H2O2 (see Recipes)
- 10% ammonium persulfate (see Recipes)
- 1.5 M Tris-HCl buffer pH 8.8 (see Recipes)
- 1.5 M Tris-HCl buffer pH 6.8 (see Recipes)
- 30% acrylamide mix solution (acrylamide bisacrylamide ratio 37.5:1) (see Recipes)
- 6% resolving gel solution (see Recipes)
- 5% stacking gel solution (see Recipes)
- 1 M Tris-HCl buffer pH 8 (see Recipes)
- 0.5 M EDTA pH 8 (see Recipes)
- Loading sample buffer (see Recipes)
- Running buffer (see Recipes)
- Ferric chloride/potassium ferricyanide solution (see Recipes)
Equipment
- 50, 125 and 150 ml sterile Erlenmeyer flasks (DWK Life Sciences, Duran®, catalog numbers: 21 216 17 , 21 216 28 , 21 990 27 )
- 2-20 µl, 20-100 µl, 100-1,000 µl Kartell pluripet micropipettes (Kartell LABWARE, catalog numbers: 13000 , 13210 , 13220 ) and 1-10 ml Acura® manual micropipette (Socorex, model: Acura® manual 825 / Acura® manual 835 )
- 50, 100 and 1,000 ml borosilicate measuring cylinders (VILABO, catalog number: 3501114 , 3501115 , 3501118 )
- Conventional incubator shaker (New Brunswick Scientific, model: G25 )
- Gyratory water bath shaker (New Brunswick Scientific, model: G76 )
- Ice maker (Brema, model: TB 551 )
- UV-Vis Spectrophotometer (Biotraza, model: 752 )
- Refrigerated centrifuge (Hanil Scientific, model: Combi 514R )
- Vibra-Cell sonicator (Sonics & Materials, model: VC500 )
- Electrophoresis cell (Bioamerica, model: DYCZ-24DNBA )
- Power supply (Bioamerica, model: DYY-6CBA )
- Freezer ultra-low temperature (Sanyo, model: MDF-U76VC )
- Autoclave (HIRAYAMA, HICLAVETM, model: HVE-50 )
- Hot air oven sterilizer (Dalvo Intrumentos, model: OHR/T )
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Pezzoni, M., Pizarro, R. A. and Costa, C. S. (2018). Detection of Catalase Activity by Polyacrylamide Gel Electrophoresis (PAGE) in Cell Extracts from Pseudomonas aeruginosa. Bio-protocol 8(11): e2869. DOI: 10.21769/BioProtoc.2869.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 活性
生物化学 > 蛋白质 > 活性
生物化学 > 蛋白质 > 电泳
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link