- EN - English
- CN - 中文
Host-regulated Hepatitis B Virus Capsid Assembly in a Mammalian Cell-free System
宿主调控的乙型肝炎病毒衣壳在无哺乳动物细胞系统中的组装
发布: 2018年04月20日第8卷第8期 DOI: 10.21769/BioProtoc.2813 浏览次数: 7093
评审: Yannick DebingAnonymous reviewer(s)
Abstract
The hepatitis B virus (HBV) is an important global human pathogen and represents a major cause of hepatitis, liver cirrhosis and liver cancer. The HBV capsid is composed of multiple copies of a single viral protein, the capsid or core protein (HBc), plays multiple roles in the viral life cycle, and has emerged recently as a major target for developing antiviral therapies against HBV infection. Although several systems have been developed to study HBV capsid assembly, including heterologous overexpression systems like bacteria and insect cells, in vitro assembly using purified protein, and mammalian cell culture systems, the requirement for non-physiological concentrations of HBc and salts and the difficulty in manipulating host regulators of assembly presents major limitations for detailed studies on capsid assembly under physiologically relevant conditions. We have recently developed a mammalian cell-free system based on the rabbit reticulocyte lysate (RRL), in which HBc is expressed at physiological concentrations and assembles into capsids under near-physiological conditions. This system has already revealed HBc assembly requirements that are not anticipated based on previous assembly systems. Furthermore, capsid assembly in this system is regulated by endogenous host factors that can be readily manipulated. Here we present a detailed protocol for this cell-free capsid assembly system, including an illustration on how to manipulate host factors that regulate assembly.
Keywords: Hepatitis B virus (乙型肝炎病毒)Background
The hepatitis B virus (HBV) is an important global human pathogen that chronically infects hundreds of millions of people worldwide and represents a major cause of viral hepatitis, liver cirrhosis, and liver cancer (Seeger et al., 2013; Trepo et al., 2014). HBV replicates its genomic DNA, a relaxed circular, partially duplex DNA (RC DNA), via reverse transcription of an RNA intermediate, the so-called pregenomic RNA (pgRNA), within a nucleocapsid (NC) (Summers and Mason, 1982; Hu and Seeger, 2015; Hu, 2016), which packages a copy of pgRNA together with the virally encoded reverse transcriptase (RT) protein (Bartenschlager and Schaller, 1992; Hu and Lin, 2009). It is within NCs that the RT converts pgRNA into RC DNA.
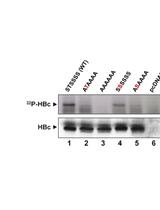
The icosahedral HBV capsid shell consists of multiple copies of a single viral protein, the HBV core (capsid) protein (HBc). HBc is composed of an N-terminal domain (NTD, aa 1-140) and a C-terminal domain (CTD, 150-183 or 185 depending on strains), which are connected by a linker region (141-149). In heterologous overexpression systems including bacteria and insect cells and in vitro assembly reactions using high concentrations of purified HBc and/or salt, NTD alone, without CTD, is sufficient for capsid assembly; thus it is also called the assembly domain (Gallina et al., 1989; Birnbaum and Nassal, 1990; Lanford and Notvall, 1990; Wingfield et al., 1995). Though not required for capsid assembly in these systems, the highly basic and arginine-rich CTD shows non-specific nucleic acid binding activities (Hatton et al., 1992) and plays important roles in viral RNA packaging (Nassal, 1992), DNA synthesis (Nassal, 1992; Yu and Summers, 1994), and nuclear import of capsids (Liao and Ou, 1995; Liu et al., 2015), all of which is further regulated by the dynamic phosphorylation state of CTD controlled by host factors (Kann and Gerlich, 1994; Kann et al., 1999; Daub et al., 2002; Ludgate et al., 2012; Liu et al., 2015). Whether CTD, and its state of phosphorylation, play a role in capsid assembly under physiological conditions remained unclear.
To study HBV capsid assembly under more physiological conditions, we have recently developed a mammalian cell-free assembly system based on the commonly used mammalian cell extract, rabbit reticulocyte lysate (RRL), in which HBc is expressed at physiological (low) concentrations (25-50 nM, monomer) and assembles into capsids under near-physiological conditions (Ludgate et al., 2016). This system allowed us to reveal an unexpected role of CTD in capsid assembly, which is further subjected to regulation by the state of CTD phosphorylation as controlled by endogenous host factors (Ludgate et al., 2016). This protocol is adapted from Ludgate et al. (2016) and more detailed information on this cell-free capsid assembly system is included, and different treatments are applied to address the roles of viral and host factors, such as RNA-binding activities of CTD and host phosphatases, in HBV capsid assembly under near-physiological conditions. This protocol will facilitate detailed studies on capsid assembly and host regulation under physiological conditions and identification of novel antiviral agents targeting HBc.
Materials and Reagents
- Pipette tips (Denville Scientific, catalog numbers: P1096-FR , P1121 , P1122 , P1126 )
- 1.5 ml microcentrifuge tube (Denville Scientific, catalog number: C2170 )
- Gloves and lab coat (Denville Scientific, catalog number: G4162 ; Medline Industries, catalog number: 83044QHW )
- Proteinase K, RNA grade (Thermo Fisher Scientific, InvitrogenTM, catalog number: 25530049 )
- Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L4509-1KG )
- Phenol solution (Sigma-Aldrich, catalog number: P4557 )
- Chloroform (Fisher Scientific, catalog number: BP1145-1 )
- Sodium acetate (Sigma-Aldrich, catalog number: S2889-1KG )
- Ethyl alcohol (EtOH) (AmericanBio, catalog number: AB00515-00100 )
- UltraPureTM DNase/RNase-Free Distilled Water (Thermo Fisher Scientific, InvitrogenTM, catalog number: 10977015 )
- Agarose (Thermo Fisher Scientific, InvitrogenTM, catalog number: 15510027 )
- TNT® Coupled Rabbit Reticulocyte Lysate (RRL) (Promega, catalog number: L4610 )
- EasyTagTM L-[35S]-Methionine (PerkinElmer, catalog number: NEG709A001MC )
- RNasin® Plus Ribonuclease Inhibitor (Promega, catalog number: N2611 )
- RNaseZapTM RNase Decontamination Solution (Thermo Fisher Scientific, InvitrogenTM, catalog number: AM9780 )
- RNase A, DNase and protease-free (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: EN0531 )
- NEBuffer 3 (New England Biolabs, catalog number: B7003S )
- Alkaline Phosphatase, Calf Intestinal (CIAP) (New England Biolabs, catalog number: M0290S )
- Tris Base (Fisher Scientific, catalog number: BP152-10 )
- Ethylenediaminetetraacetic acid, EDTA (Sigma-Aldrich, catalog number: E5134 )
- Sodium fluoride (Sigma-Aldrich, catalog number: S7920 )
- Sodium pyrophosphate tetrabasic decahydrate (Sigma-Aldrich, catalog number: S6422 )
- β-Glycerophosphate (Sigma-Aldrich, catalog number: G6251 )
- Sodium orthovanadate (Sigma-Aldrich, catalog number: S6508 )
- cOmpleteTM, EDTA-free Protease Inhibitor Cocktail (Roche Diagnostics, catalog number: 04693132001 )
- TE buffer (see Recipes)
- 10x phosphatase inhibitors (PPI) (see Recipes)
- 25x protease inhibitor (see Recipes)
Equipment
- Pipettes (Gilson, P1000, P200, P20, P2)
- Fume hood (e.g., Protector Xstream Laboratory Hood, Labconco)
- 30 °C/37 °C Oven (SciGene, Robbins Scientific, model: Model 400 )
- Microcentrifuge (Fisher Scientific, FisherbrandTM, model: accuSpinTM Micro 17 )
- Spectrophotometer (Thermo Fisher Scientific, Thermo ScientificTM, model: NanoDropTM1000 )
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Liu, K. and Hu, J. (2018). Host-regulated Hepatitis B Virus Capsid Assembly in a Mammalian Cell-free System. Bio-protocol 8(8): e2813. DOI: 10.21769/BioProtoc.2813.
分类
微生物学 > 异源表达系统 > 体外翻译
微生物学 > 微生物生物化学 > 蛋白质 > 修饰
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link