- EN - English
- CN - 中文
In vivo Analysis of Cyclic di-GMP Cyclase and Phosphodiesterase Activity in Escherichia coli Using a Vc2 Riboswitch-based Assay
使用基于Vc2 Riboswitch的测定法体内分析大肠杆菌中环状di-GMP环化酶和磷酸二酯酶活性
(*contributed equally to this work) 发布: 2018年03月05日第8卷第5期 DOI: 10.21769/BioProtoc.2753 浏览次数: 7835
评审: Dennis NürnbergKanika GeraAnonymous reviewer(s)

相关实验方案
使用 Seahorse XFe96 细胞外通量分析仪实时分析弓形虫寄生虫的线粒体电子传递链功能
Jenni A. Hayward [...] Giel G. van Dooren
2022年01月05日 4062 阅读
Abstract
Cyclic di-guanosine monophosphate (c-di-GMP) is a ubiquitous second messenger that regulates distinct aspects of bacterial physiology. It is synthesized by diguanylate cyclases (DGCs) and hydrolyzed by phosphodiesterases (PDEs). To date, the activities of DGC and PDE are commonly assessed by phenotypic assays, mass spectrometry analysis of intracellular c-di-GMP concentration, or riboswitch-based fluorescent biosensors. However, some of these methods require cutting-edge equipment, which might not be available in every laboratory. Here, we report a new simple, convenient and cost-effective system to assess the function of DGCs and PDEs in E. coli. This system utilizes the high specificity of a riboswitch to c-di-GMP and its ability to regulate the expression of a downstream β-galactosidase reporter gene in response to c-di-GMP concentrations. In this protocol, we delineate the construction of this system and its use to assess the activity of DGC and PDE enzymes.
Keywords: Cyclic di-guanylate monophosphate (c-di-GMP) (环鸟苷酸单磷酸酯(c-di-GMP))Background
Cyclic-di-GMP is an important and ubiquitous second messenger in bacteria, which regulates a variety of processes, such as motility-to-sessility transition, biofilm formation, virulence, and cell cycle progression (Römling et al., 2013). The GG(D/E)EF domain has diguanylate cyclase (DGC) activity and it is responsible for the synthesis of c-di-GMP from two GTPs, which is a two-step reaction with 5’-pppGpG as intermediate and two molecules of pyrophosphate as by-products (Ryjenkov et al., 2005). Phosphodiesterases (PDE) with an EAL or an HD-GYP domain hydrolyze c-di-GMP into linear 5’-pGpG (Schmidt et al., 2005) and GMP (Ryan et al., 2006), respectively.
Several tools have been developed to monitor intracellular cyclic di-nucleotide levels and to identify proteins involved in cyclic di-nucleotide signaling, for example, protein-based fluorescence resonance energy transfer (FRET) biosensor (Christen et al., 2010), riboswitch-based fluorescent biosensor (Kellenberger et al., 2015), and riboswitch-based dual-fluorescence reporter (Zhou et al., 2016). However, these tools monitor altered fluorescence of reporters and require the access to flow cytometry or fluorescence microscopy. Here, we report the development of an alternative assay to monitor the intracellular c-di-GMP concentration, namely by monitoring the alteration in β-galactosidase activity in agar-growing cells. For that, the Vc2 riboswitch (Sudarsan et al., 2008) is fused translationally to lacZY and integrated into the chromosome of E. coli strain TOP10. Vc2 is an ‘off’ riboswitch from Vibrio cholerae and thus down-regulates the expression of β-galactosidase when c-di-GMP is bound (Figure 1). The stable integration into the Tn7 attachment site in the chromosome of E. coli avoids copy number effects and eliminates the need to use an antibiotic resistance marker. Changes in c-di-GMP levels are subsequently translated to the alteration in β-galactosidase expression, which is reflected by the color change of the colony growing on an agar plate containing 5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal). This assay can be used, for instance, to reveal the function of proteins under physiological condition and to assess the enzymatic activity of proteins that are challenging to be purified and tested in vitro. However, the Vc2-based assay described here is a qualitative assessment of the change in intracellular c-di-GMP concentration. Quantification is not crucial for a screening assay, but would be advantageous in, for example, measuring the activity of enzymes. We have demonstrated that the Vc2-based assay can be exploited to verify the activity of both DGCs and PDEs in vivo (El Mouali et al., 2017).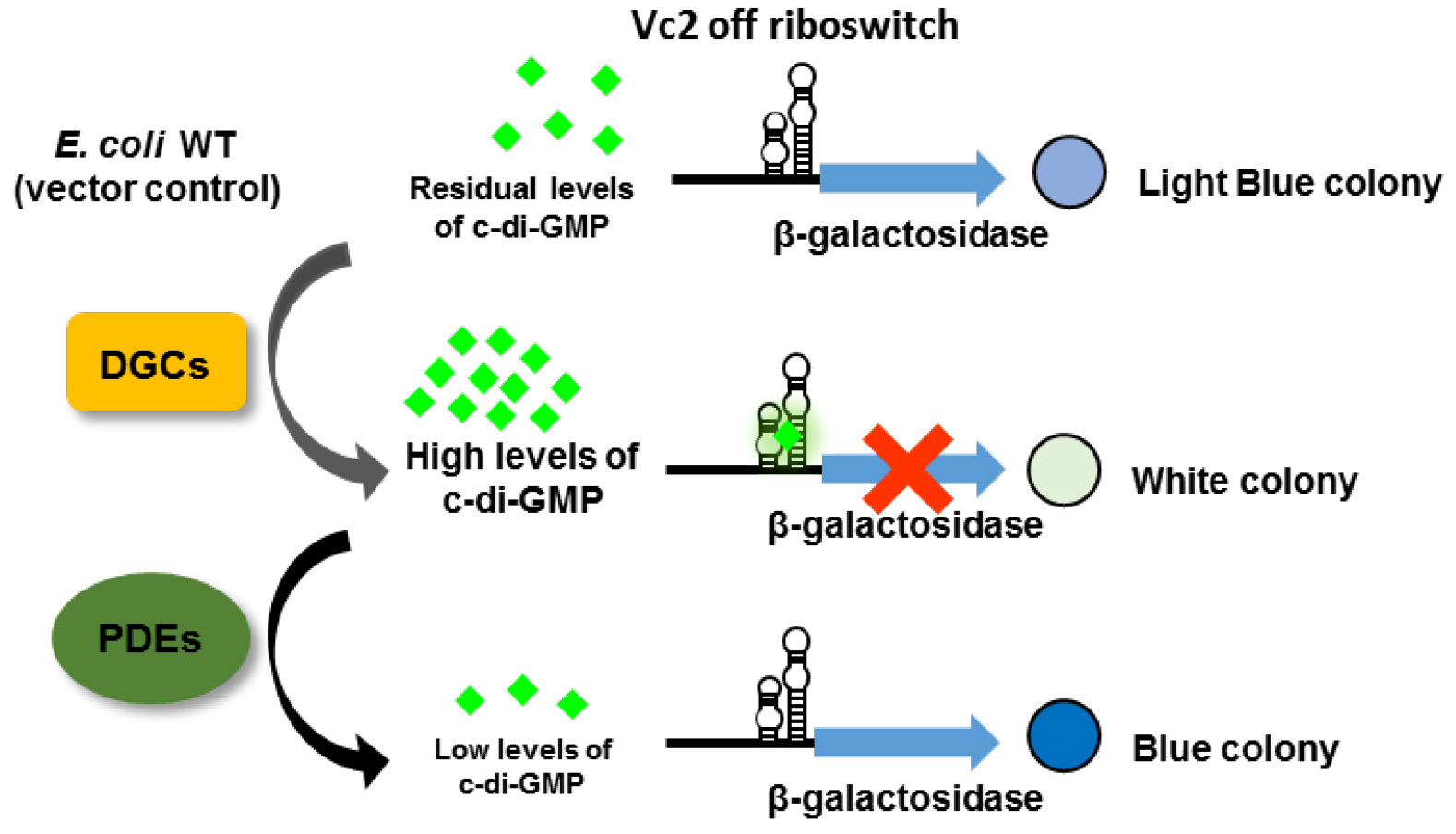
Figure 1. The principle of a riboswitch-based screening system. The Vc2 riboswitch is located upstream of the β-galactosidase open reading frame to control its expression in response to the variation in c-di-GMP concentration. When c-di-GMP is present in high abundance due to the overexpression of DGCs, the expression of β-galactosidase is down-regulated resulting in a white colony on an X-gal containing plate. In contrast, when PDEs are overexpressed, generating low intracellular c-di-GMP concentration, the colony is blue. In E. coli TOP10 wild type cells, there are residual amounts of c-di-GMP, resulting in a light blue colony (adapted from El Mouali et al., 2017).
Materials and Reagents
- PCR tubes
- Petri dish 92 x 16 mm (SARSTEDT, catalog number: 82.1472.001 )
- Syringe filters, 0.2 μm pore size (SARSTEDT, catalog number: 83.1826.001 )
- Microcentrifuge tubes of 1.5 ml (SARSTEDT, catalog number: 72.690.001 )
- Pipette tips (1,000 μl: SARSTEDT, catalog number: 70.762.100 ; 1-200 μl: Corning, catalog number: 4804 ; 0.1-10 μl: Gilson, catalog number: F171103 )
- Spectrophotometer cuvettes (SARSTEDT, catalog number: 67.742 )
- 3-part disposable HSW SOFT-JECT® syringes (5 ml: Henke Sass Wolf, catalog number: 5050.X00V0 ; 10 ml: Henke Sass Wolf, catalog number: 5100.X00V0 )
- Aluminum foil
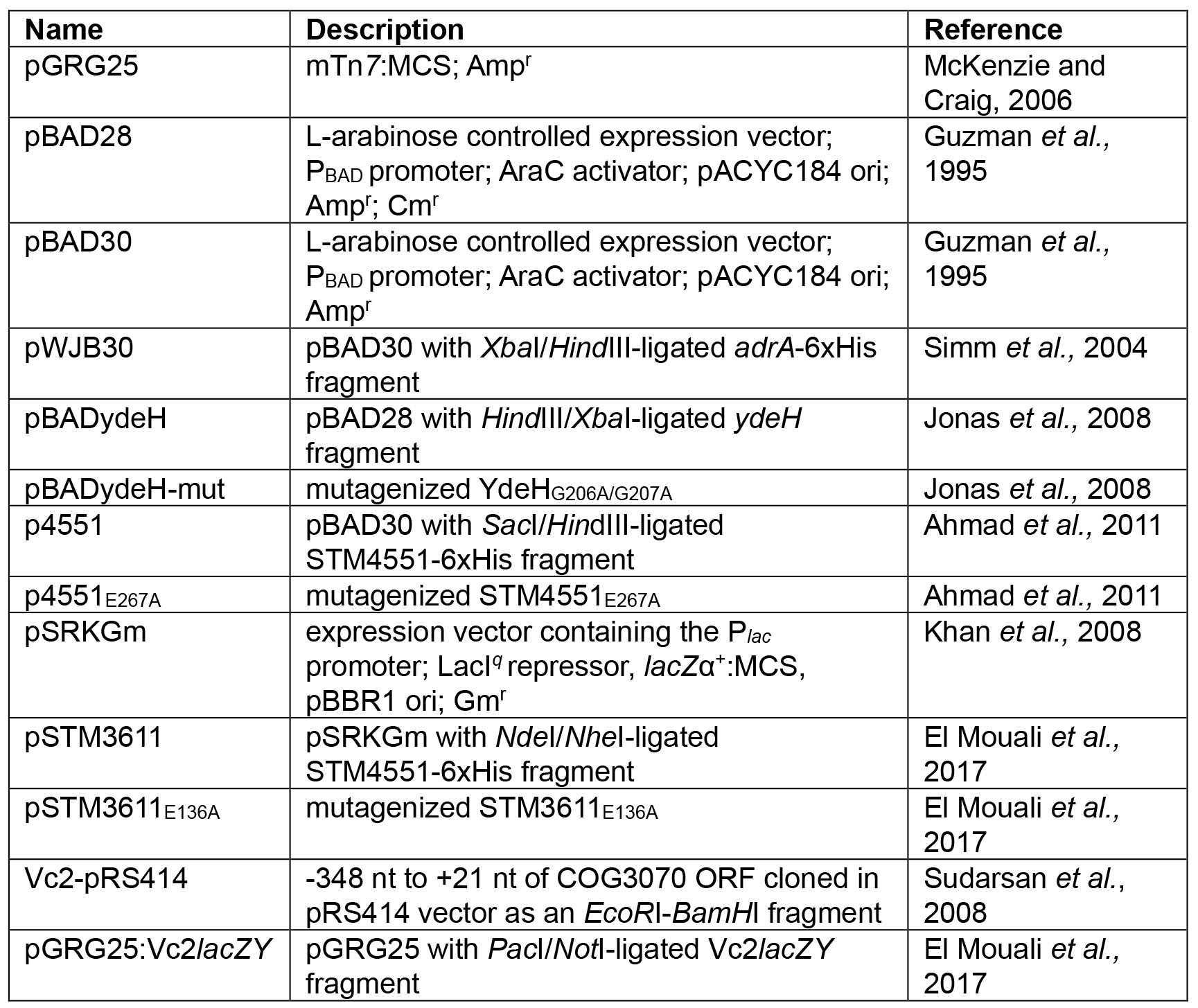
- Plasmids used in this study:
- Calcium chloride competent E. coli TOP10 cells (Hanahan, 1983)
- Primers used in this study (ordered from Sigma-Aldrich):

aThe restriction sites are underlined. - GenElute Plasmid Miniprep Kit (Sigma-Aldrich, catalog number: PLN350 )
- Sterile distilled, deionized water (diH2O)
- Phusion High-fidelity DNA polymerase (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: F530S )
- dNTP mix (10 mM each) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: R0192 )
- Agarose (Sigma-Aldrich, catalog number: A9539 )
- SmartLadder for DNA: 200-10,000 bp (Eurogentec, catalog number: MW-1700-10 )
- GelRedTM nucleic acid gel stain (Biotium, catalog number: 41003 )
- NotI restriction enzyme (New England Biolabs, catalog number: R0189S )
- PacI restriction enzyme (New England Biolabs, catalog number: R0547S )
- PCR purification kit (QIAGEN, catalog number: 28106 )
- Rapid DNA ligase kit (Roche Diagnostics, catalog number: 10716359001 )
- Taq DNA polymerase with standard Taq buffer (New England Biolabs, catalog number: M0273S )
- Sodium chloride (Sigma-Aldrich, catalog number: S7653 )
- Tryptone (BD, BactoTM, catalog number: 211705 )
- Yeast extract (BD, BactoTM, catalog number: 212750 )
- Agar (BD, DifcoTM, catalog number: 281230 )
- Tris base (Sigma-Aldrich, catalog number: 93350 )
- Glacial acetic acid (Sigma-Aldrich, catalog number: ARK2183 )
- EDTA (Sigma-Aldrich, catalog number: E9884 )
- Ampicillin sodium salt (Sigma-Aldrich, catalog number: A9518 )
- Gentamicin sulfate salt (Sigma-Aldrich, catalog number: G3632 )
- L-(+)-arabinose (Sigma-Aldrich, catalog number: A3256 )
- Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma-Aldrich, catalog number: I6758 )
- Dimethyl sulfoxide (Sigma-Aldrich, catalog number: D8418 )
- 5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) (Roche Diagnostic, catalog number: 10703729001 )
- LB (Luria-Bertani) medium (see Recipes)
- LB (Luria-Bertani) agar (see Recipes)
- TAE buffer (see Recipes)
- 100 mg/ml ampicillin stock (see Recipes)
- 30 mg/ml gentamicin stock (see Recipes)
- 100 mM IPTG stock (see Recipes)
- 20 mg/ml X-gal stock (see Recipes)
Equipment
- Erlenmeyer flasks (50 ml/100 ml/250 ml)
- Pipettes [e.g., PIPETMAN® P20 (Gilson, catalog number: F123600 ), P200 (Gilson, catalog number: F123601 ), or P1000 (Gilson, catalog number: F123602 )]
- SureCycler 8800 thermal cycler (Agilent Technologies, model: SureCycler 8800 , catalog number: G8800A)
- HeraeusTM PicoTM 17 Microcentrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: HeraeusTM PicoTM 17 , catalog number: 75002410)
- Mini-Sub® cell GT Horizontal Electrophoresis System (Bio-Rad Laboratories, catalog number: 1704406 )
- 15-well comb (Bio-Rad Laboratories, catalog number: 1704464 )
- Sub-Cell GT UV-Transparent Mini-Gel Tray (Bio-Rad Laboratories, catalog number: 1704436 )
- Electrophoresis power supply
- Heraeus® microbiological incubator (Thermo Fisherer Scientific, Thermo ScientificTM, model: Heraeus® microbiological incubator )
- Multitron Standard shaker (INFORS HT)
- Gel DocTM XR+ Gel Documentation System (Bio-Rad Laboratories, catalog number: 1708195 )
- BioPhotometer (Eppendorf, model: 6131 )
- NanoDropTM 2000c Spectrophotometers (Thermo Fisher Scientific, Thermo ScientificTM, model: NanoDropTM 2000c , catalog number: ND-2000C)
- Thermomixer compact (Eppendorf, model: 5350 )
- Digital camera
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Liu, Y., Kim, H. and Römling, U. (2018). In vivo Analysis of Cyclic di-GMP Cyclase and Phosphodiesterase Activity in Escherichia coli Using a Vc2 Riboswitch-based Assay. Bio-protocol 8(5): e2753. DOI: 10.21769/BioProtoc.2753.
分类
微生物学 > 微生物信号传导 > 第二信使
微生物学 > 微生物细胞生物学 > 基于细胞的分析方法 > 报告基因实验
分子生物学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link