- EN - English
- CN - 中文
Chromatin Affinity Purification (ChAP) from Arabidopsis thaliana Rosette Leaves Using in vivo Biotinylation System
使用体内生物素化系统进行拟南芥莲座叶染色质亲和纯化(ChAP)
发布: 2018年01月05日第8卷第1期 DOI: 10.21769/BioProtoc.2677 浏览次数: 9455
评审: Amey G RedkarAnonymous reviewer(s)
Abstract
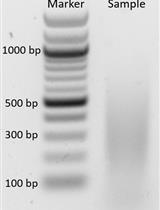
Chromatin Affinity Purification (ChAP) is widely used to study chromatin architecture and protein complexes interacting with DNA. Here we present an efficient method for ChAP from Arabidopsis thaliana rosette leaves, in which in vivo biotinylation system is used. The chromatin is digested by Micrococcal Nuclease (MNase), hence the distribution of nucleosomes is also achieved. The in vivo biotinylation system was initially developed for Drosophila melanogaster (Mito et al., 2005), but the presented protocol has been developed specifically for Arabidopsis thaliana (Sura et al., 2017).
Keywords: Chromatin affinity purification (染色质亲和纯化)Background
Chromatin Immunoprecipitation (ChIP) became one of the most important and commonly used technique to study chromatin structure and organization. However, it requires good-quality antibodies, which will not cross-react with non-specific targets. This is relatively difficult to achieve in plants, which contain cell wall and are rich in photosynthesis-related compounds and proteins frequently causing cross-reactivity problems. On the other hand, obtaining stable transgenic organisms is a routine and easy strategy in plants. For these reasons most plant researchers choose gene tagging, where fusion proteins are obtained and used to study chromatin in an approach alternative to ChIP, that is Chromatin Affinity Purification (ChAP). The ChAP technique has proven to be extremely effective in plant chromatin studies (Zentner and Henikoff, 2014). Moreover, it is usually cheaper than classical ChIP as it does not require generation of antibodies, and is often more effective than ChIP as tags are recognized with higher affinity than antibodies raised directly against proteins of interests. One disadvantage of ChAP is that it cannot be used to study post-translational histone modifications. In the presented protocol, proteins are tagged with a short, Biotin ligase recognition peptide (BLRP), which is in vivo biotinylated by Escherichia coli BirA biotin ligase (de Boer et al., 2003). Consequently, the tagged protein is purified using streptavidin-based purification systems (e.g., Dynabeads M-280 Streptavidin, Invitrogen). As streptavidin has an extraordinarily high affinity for biotin (dissociation constant on the order of 10-14 mol/L), the binding of biotin to streptavidin is one of the strongest non-covalent interactions known in nature (Green, 1975). Alternatively, the presented protocol can be successfully applied for ChAP of proteins labeled with other tags (e.g., MYC, GFP, HA, FLAG) if a suitable system for final purification is used.
Materials and Reagents
- Standard pipette tips
- 15 ml and 50 ml conical centrifuge tubes
- Nylon mesh (pore size 80 μm, Membrane Solutions, catalog number: MENY090080 )
- Paper towels
- Miracloth (Merck, Calbiochem, catalog number: 475855 )
- 1.5-2.0 ml microcentrifuge tubes
- 2 ml and 1.5 ml low retention tubes (Maxymum recovery microtubes; Corning, Axygen®, catalog numbers: MCT-200-L-C and MCT-150-L-C )
- Plant material: A. thaliana plants expressing E. coli BirA biotin ligase under control of a strong promoter (e.g., Arabidopsis thaliana Act8) with a gene of interest fused with Biotin ligase recognition peptide (BLRP) (de Boer et al., 2003; Mito et al., 2005)
Note: Plants could be at different developmental stages from one-week-old seedlings to adult, prior flowering. In general, the younger material, the higher efficiency of chromatin isolation. Moreover, younger plants provide higher homogeneity of the tissue used for chromatin extraction and lower variation between replicates. BirA biotin ligase could be expressed from different promoters, however, expression should be kept at high levels. Other possible promoters include Ubiquitin 10 promoter (UBQ10) and Cauliflower Mozaic Virus (CaMV) 35S promoter. Inducible systems e.g., dexamethasone (DEX)-inducible, can be potentially also used, however this was not tested in our laboratory. - 2 M glycine (made of Glycine, Sigma-Aldrich, catalog number: 33226 )
Note: This product has been discontinued. - Liquid nitrogen, ice
- 1 M CaCl2 (made of CaCl2, Sigma-Aldrich, catalog number: C5670 )
- Micrococcal nuclease (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: EN0181 )
- 0.5 M EGTA (made of EGTA, BioShop, catalog number: EGT101 )
- 0.5 M EDTA (made of EDTA, BioShop, catalog number: EDT001 )
- RNase A (Sigma-Aldrich, catalog number: R4642 )
- Proteinase K (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: EO0491 )
- 3 M sodium acetate pH 5.2 (made of CH3COONa, INC Biomedicals Inc.)
- Glycogen (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: R0561 )
- Ethanol (Avantor Performance Materials, catalog number: BA6420113 )
- Agarose (BioShop, catalog number: AGA001.1 )
- Gene Ruler 1 kb, GR 100 bp and GR 100 bp plus (Thermo Fisher Scientific, Thermo ScientificTM, catalog numbers: SM0311 , SM0241 and SM0321 , respectively)
- Dynabeads M-280 Streptavidin (Thermo Fisher Scientific, InvitrogenTM, catalog number: 11206D )
- Chelex 100 (Bio-Rad Laboratories, catalog number: 1421253 )
- Tris (BioShop, catalog number: TRS001.5 )
- 1 M Tris-HCl pH 8 (made of Tris)
- 1 M Tris-HCl pH 6.8 (as above)
- Acetic acid (Avantor Performance Materials, catalog number: BA8760114 )
- Sucrose (Sigma-Aldrich, catalog number: S0389 )
- Phenylmethylsulfonyl fluoride (Sigma-Aldrich, catalog number: P7626 )
- Formaldehyde (Sigma-Aldrich, catalog number: F8775-500ML or BioShop, catalog number: FOR201 )
- Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266 )
- Triton X-100 (Carl Roth, catalog number: 3051.3 )
- β-Mercaptoethanol (BioShop, catalog number: MER002 )
- Spermine (Sigma-Aldrich, catalog number: 85590-5G )
- 5 M NaCl (made of NaCl, Avantor Performance Materials, catalog number: 794121116 )
- Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L3771 )
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761 )
- Phenol (Sigma-Aldrich, catalog number: P4557 )
- Chloroform (Avantor Performance Materials, catalog number: BA6420113 )
- Isoamyl alcohol (Avantor Performance Materials, catalog number: 485560111 )
- Maxima Sybr Green/ROX qPCR Master Mix (2x) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: K0223 )
- Protease inhibitor cocktail (Sigma-Aldrich, catalog number: P8465 )
- 1x TE buffer (see Recipes)
- 1x TAE buffer (see Recipes)
- Cross-linking buffer (see Recipes)
- Honda buffer (see Recipes)
- TNE buffer (see Recipes)
- Extraction buffer (see Recipes)
- Pellet extraction buffer (see Recipes)
- Phenol/Chloroform/Isoamyl alcohol for DNA extraction (see Recipes)
- ChIP Dilution Buffer (see Recipes)
- Binding/Washing Buffer (see Recipes)
Equipment
- Pipettes (Gilson, model: Pipetman® Neo or PZ HTL, model: Discovery Comfort )
- Fume hood
- Desiccator connected to the pump
- Mortars and pestles
- Small funnels
- Scale (KERN & SOHN, catalog number: EG 220-3NM )
- Rotator (Cole-Parmer, Stuart, model: SB3 )
- Centrifuges:
Thermo Fisher Scientific, Thermo ScientificTM, model: HeraeusTM MultifugeTM X1R, catalog number: 75004250
Thermo Fisher Scientific, Thermo ScientificTM, model: HeraeusTM BiofugeTM StratosTM Centrifuge, catalog number: 75005282
Beckman Coulter, model: Allegra® X-30R Centrifuge, catalog number: A99471
Eppendorf, model: MiniSpin® plus , catalog number: 0040262
Gilson, model: CmC Lab CAPSULEFUGE PMC-880 - Thermomixer (Eppendorf, model: Thermomixer comfort , catalog number: 5355)
- Magnetic separator (Thermo Fisher Scientific, model: DynaMagTM-2, catalog number: 12321D )
- See Saw Rocker (Cole-Parmer, Stuart, model: SSL4 )
- Thermoblock (Grant Instruments, model: QBA2 )
- Vortex (Scientific Industries, model: Vortex-Genie 2 , catalog number: SI-0256)
- Systems for DNA electrophoresis (Mini-Sub Cell and Wide Mini-Sub Cell GT Complete Systems, Bio-Rad Laboratories, catalog numbers: 1640300 and 1640301 , respectively)
- Real-Time PCR System (Thermo Fisher Scientific, Applied BiosystemsTM, model: 7900HT Fast Real-Time PCR System, catalog number: 4329001 )
Software
- 7900HT Fast Real-Time PCR System Software: SDSv2.4 (available on Thermo Scientific website)
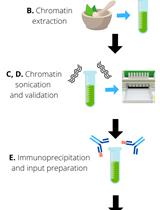
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Sura, W. and Ziolkowski, P. A. (2018). Chromatin Affinity Purification (ChAP) from Arabidopsis thaliana Rosette Leaves Using in vivo Biotinylation System. Bio-protocol 8(1): e2677. DOI: 10.21769/BioProtoc.2677.
分类
植物科学 > 植物分子生物学 > 染色质
分子生物学 > DNA > DNA-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link