- EN - English
- CN - 中文
Isolation, Culture and Differentiation of Adult Hippocampal Precursor Cells
成年海马前体细胞的分离,培养和分化
(*contributed equally to this work) 发布: 2017年11月05日第7卷第21期 DOI: 10.21769/BioProtoc.2603 浏览次数: 20112
评审: Anonymous reviewer(s)
Abstract
There are two neurogenic niches in the adult mammalian brain: the subventricular zone of the lateral ventricle and the subgranular zone of the hippocampal dentate gyrus. Cells from these areas can be isolated and maintained in vitro, using two different culture systems to assess their potential regarding proliferation and differentiation in a reductionist model. While the neurosphere assay is primarily performed to directly study the proliferative and differentiation potential of cells in individual brains, the monolayer culture allows single cell analysis in a rather homogeneous cell population. Here, we describe the isolation, culturing methods and differentiation of neural precursor cells in both systems.
Keywords: Neuroscience (神经科学)Background
In the mammalian brain, adult neural stem cells reside in two main neurogenic niches, the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and the lateral ventricle of the subventricular zone (SVZ), that allow the generation of new neurons in the adult brain. Neural precursor cells from the neurogenic niches can be isolated and cultured in vitro to model cellular processes, especially proliferation and differentiation. Two standard culture systems, the adherent monolayer culture (Palmer et al., 1995; Ray et al., 1995) and the neurosphere assay (Reynolds and Weiss, 1992 and 1996), both introduced in the 1990s, represent valuable tools to study neural progenitor cell biology in vitro.
Depending on the research question, each system has advantages and disadvantages that should be considered carefully before choosing one or the other culture method. In adherent monolayer cultures cells grow rather isolated and form more homogeneous cultures. Monolayers allow the direct investigation and monitoring of neural precursor cells at the single cell level. Characteristics like morphology, proliferation and differentiation under controlled conditions, can easily be analysed and visualised. However, compared to neurosphere cultures, cells cultured as monolayer represent a more reductionist model as the cells grow with fewer cell-to-cell contacts that are usually present in the niche.
Neurosphere cultures are free-floating aggregate cultures that are easy to obtain from adult tissue. Primary neurospheres are more heterogeneous and presumably represent a more niche-like environment. Neurospheres can be used to model the interaction of different cell types and allow relative comparisons of precursor cell number and potential, but does not allow absolute conclusions about stem cell numbers in vivo. Also, the sphere-forming capacity is not identical to ‘stemness’.
This protocol describes the detailed workflow of the generation and analysis of adult neural precursor cultures as neurospheres and monolayers from both neurogenic regions, the SVZ and the DG. The protocol represents an optimized version of our previously published protocols that have been successfully applied to many research projects within our group and by other groups (Babu et al., 2011; Walker and Kempermann, 2014; Ehret et al., 2016; Hörster et al., 2017).
Materials and Reagents
- Animals
Mice: C57BL/6J (8 weeks old)
Note: We recommend three to four mice for establishing a monolayer cell culture. For neurosphere assay experiments we recommend one mouse per 96-well plate. - General materials and reagents
- Centrifugation tubes 15 ml and 50 ml
- Reaction tubes 1.5 ml
- Parafilm
- 70% ethanol
- Double distilled water (ddH2O)
- 1x phosphate-buffered saline (PBS)
- DMEM/F-12 without glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 21331020 )
- 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer pH 7.4
- PFA (Merck, catalog number: 1040051000 )
- Sodium dihydrogen phosphate (Merck, catalog number: 1063421000 )
- Disodium phosphate dihydrate (Acros Organics, catalog number: 343810025 )
- Sodium hydroxide (NaOH) (Carl Roth, catalog number: 6771 )
- PFA (Merck, catalog number: 1040051000 )
- Growth media (see Recipes)
- Neurobasal® medium (Thermo Fisher Scientific, GibcoTM, catalog number: 21103049 )
- B-27® supplement (50x) (Thermo Fisher Scientific, GibcoTM, catalog number: 17504044 )
- Pen/Strep 100,000 U/ml (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- GlutaMAXTM supplement (100x stock) (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061 )
- Neurobasal® medium (Thermo Fisher Scientific, GibcoTM, catalog number: 21103049 )
- Fire polished pipettes
- Glass Pasteur pipette (1 mm diameter)
- Glass Pasteur pipette (1 mm diameter)
- Coating
- Poly-D-Lysine hydrobromide (PDL) (Sigma-Aldrich, catalog number: P7280 )
- Laminin (Roche Diagnostics, catalog number: 11243217001 )
- Poly-D-Lysine hydrobromide (PDL) (Sigma-Aldrich, catalog number: P7280 )
- Brain dissection
Petri dishes (10 cm diameter) - SVZ tissue dissociation
- Petri dishes (6 cm diameter)
- Scalpel (#10) (Fisher Scientific, catalog number: 11995756)
Manufacturer: B. Braun Melsungen, catalog number: 5518059 . - Falcon® 40 µm cell strainer (Corning, Falcon®, catalog number: 352340 )
- 0.05% trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25300054 )
- Trypsin inhibitor containing DNAse I (see Recipes)
- Trypsin inhibitor (Sigma-Aldrich, catalog number: T6522 )
- DNase I (Roche Diagnostics, catalog number: 10104159001 )
- Trypsin inhibitor (Sigma-Aldrich, catalog number: T6522 )
- Petri dishes (6 cm diameter)
- DG tissue dissociation
- Petri dishes (6 cm diameter)
- Falcon® 40 µm cell strainer (Corning, Falcon®, catalog number: 352340 )
- Neural tissue dissociation kit (P) (Miltenyi Biotech, catalog number: 130-092-628 )
- Beta-mercaptoethanol (Sigma-Aldrich, catalog number: M7522 )
Note: This product has been discontinued. - Hank’s buffered salt solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14175 )
- Petri dishes (6 cm diameter)
- Monolayer culture
- Tissue culture flasks (T25 and T75)
- 24-well tissue culture plates (growth-enhance treated, gamma-sterilized, free of pyrogens, free of RNA/DNA, DNase, RNase)
- Coverglass slips 12 mm (Fisher Scientific, catalog number: 12-545-82 )
Note: This product has been discontinued. - Heparin (MP Biomedicals, catalog number: 0210193125 )
- Human EGF (PeproTech, catalog number: AF-100-15 )
- Human FGF2 (PeproTech, catalog number: 100-18B )
- Accutase solution (Sigma-Aldrich, catalog number: A6964 )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418 )
- Normal donkey serum (Jackson ImmunoResearch Laboratories, catalog number: 017-000-121 )
- Trypan blue solution, 0.4% (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061 )
- BrdU (Sigma-Aldrich, catalog number: B5002 )
- Freezing mix (see Recipes)
- Antibody solution (see Recipes)
- Tissue culture flasks (T25 and T75)
- Neurosphere assay
- Petri dishes (10 cm diameter)
- 96-well tissue culture plates (growth-enhance treated, gamma-sterilized, free of pyrogens, free of RNA/DNA, DNase, RNase)
- 24-well tissue culture plates (growth-enhance treated, gamma-sterilized, free of pyrogens, free of RNA/DNA, DNase, RNase)
- Coverglass slips 12 mm (Fisher Scientific, catalog number: 12-545-82 )
- Heparin (MP Biomedicals, catalog number: 0210193125 )
- Human EGF (Peprotech, catalog number: AF-100-15 )
- Human FGF2 (PeproTech, catalog number: 100-18B )
- Blocking solution (see Recipes)
- Petri dishes (10 cm diameter)
- Staining reagents
- Microscope slides SuperFrost® (VWR, Thermo Scientific, catalog number: 631-0706 )
- Triton® X-100 (Carl Roth, catalog number: 3051 )
- 1 N HCl (from 37% stock solution) (Sigma-Aldrich, catalog number: 435570 )
Note: This product has been discontinued. - 0.9% NaCl
- Normal donkey serum (Jackson ImmunoResearch Laboratories, catalog number: 017-000-121 )
- Hoechst 33342 (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 62249 )
- Primary antibodies (see Table 1)
- Aqua-Poly/Mount (Polysciences, catalog number: 18606 )
- Borate buffer (see Recipes)
- Boric acid (Carl Roth, catalog number: 6943.1 )
- Sodium hydroxide (NaOH) (Carl Roth, catalog number: 6771 )
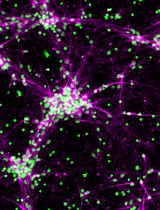
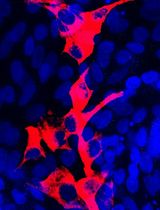
Table 1. Primary antibodies for immunocytochemistryAntibody Host Clone Isotype Company Catalog number β-III-tubulin (β-tubulin) Mouse 5G8 IgG1 Promega G7121 5’-bromo-2’-deoxyuridine (BrdU) Rat BU1/75 (ICR1) IgG2a Bio-Rad Laboratories OBT0030 Glial fibrillary acidic protein (GFAP) Rabbit polyclonal - Agilent Technologies Z0334 Map2ab Mouse AP-20 IgG1 Sigma-Aldrich M1406 Nestin Mouse 25/NESTIN IgG1, κ BD 611658 Oligodendrocyte marker 4 (O4) Mouse O4 IgM R&D Systems MAB1326 Sox2 Rabbit polyclonal - Merck AB5603
- Boric acid (Carl Roth, catalog number: 6943.1 )
- Microscope slides SuperFrost® (VWR, Thermo Scientific, catalog number: 631-0706 )
Equipment
- Bunsen burner
- Autoclave
- Dissection tools
- Scissors
- Small spatula (Fine Science Tools, catalog number: 10093-13 )
- Curved forceps (Fine Science Tools, model: Dumont #7, catalog number: 11271-30 )
- Angled forceps (Fine Science Tools, model: Dumont #5-45, catalog number: 11253-25 )
- 27 G ¾ needle (B. Braun Melsungen, catalog number: 4657705-02 )
- Scissors
- Vacuum pump
- Stereo microscope (Olympus, model: SZ61 )
- Inverted microscope (Olympus, model: CKX42 )
- Centrifuge with swing bucket rotor for 15 ml and 50 ml centrifuge tubes (Eppendorf, model: 5430 R )
- Incubator at 37 °C with 5% CO2
- Sterile laminar flow hood
- Hemocytometer (Neugebauer improved)
- Fluorescence microscope (ZEISS, model: Axio Imager.M2 )
- Freezing containers, Mr. FrostyTM (Thermo Fisher Scientific, Thermo ScientificTM, model: Mr. FrostyTM, catalog number: 5100-0001 )
- 37 °C water bath
- -80 °C freezer
- Pipettes
- Multistepper pipette (and tips)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Bernas, S. N., Leiter, O., Walker, T. L. and Kempermann, G. (2017). Isolation, Culture and Differentiation of Adult Hippocampal Precursor Cells. Bio-protocol 7(21): e2603. DOI: 10.21769/BioProtoc.2603.
分类
神经科学 > 细胞机理 > 细胞分离和培养
干细胞 > 成体干细胞 > 神经干细胞
细胞生物学 > 细胞分离和培养 > 细胞分化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link