- EN - English
- CN - 中文
Xenograft Mouse Model of Human Uveal Melanoma
人葡萄膜黑色素瘤异种移植小鼠模型
发布: 2017年11月05日第7卷第21期 DOI: 10.21769/BioProtoc.2594 浏览次数: 9361
评审: Anonymous reviewer(s)
Abstract
Uveal melanoma (UM) is a malignant intraocular tumor in adults. Metastasis develops in almost half of the patients and over 90% of the metastases are in the liver. With the advances in molecular targeting therapy for melanoma, a proper metastasis animal model is of increasing importance for testing the accuracy and effectiveness of systemic therapies. Here, we describe a xenograft model for mimicking human UM liver metastasis by injecting human UM cells into the vitreous cavity in nude mice. The athymic nude mice are immunocompromised and suitable for xenograft tumor growth and metastasis, and intravitreal injection of cells is a quicker and easier operation under a binocular scope, thereby it is simple and effective to test human UM growth and metastasis.
Keywords: Human uveal melanoma (人葡萄膜黑色素瘤)Background
UM is the most common primary intraocular tumor in adults, with an incidence rate varying from 5 to 10 cases per million in the world (Singh et al., 2011). Almost half of the patients develop metastasis within 15 years from initial diagnosis even after treatment or/and removal of primary tumor (Kujala et al., 2003; Weis et al., 2016). In over 4% of the patients, micrometastasis already exists at the time of diagnosis (Finger et al., 2005). The number might be underestimated because of limitations in detection of early UM. Current medical treatments such as enucleation, plaque brachytherapy, proton beam irradiation have been successful in removing or repressing early focal ocular UMs (Dogrusoz et al., 2017). But in general, UM is resistant to the standard chemotherapies and to date no effective systemic treatment is available for metastatic lesions (Goh and Layton, 2016; Carvajal et al., 2017). Although various advances have been made in UM treatment over decades, one-year survival rate after metastasis remains unchanged at 10-15% (Woodman, 2012).
UM arises from uveal melanocytes. Enriched with blood supply and lack of ocular lymph ducts, UM cells mainly spread to distant organs hematogenously. As a result, 95% of UM metastases have a predilection for the liver (Woodman, 2012). The mechanism underlying metastatic transformation of UM is still not clear. Since the liver metastasis is the leading cause of UM-related death (Collaborative Ocular Melanoma Study, 2011), current studies have been focused on the mechanism and molecular targeted prevention of tumor metastasis. Based on the metastatic proclivity, UMs are divided into two categories: class 1 and 2. Class 2 UMs are more inclined to metastasis with primitive stem cell-like gene expression pattern (Harbour and Chao, 2014). The activation of RB/P53, PI3K/AKT and MAPK signaling pathways leads to tumor overgrowth and anti-apoptosis (Coupland et al., 2013; Reichstein, 2017). 85% of primary and metastatic UMs are presented with gain-function mutations in either of two G-protein genes, GNAQ and GNA11 (Shoushtari and Carvajal, 2014). Loss of chromosome 3 or loss-function mutation in BAP1 gene indicates a poor prognosis and metastatic UM (Damato et al., 2011; van Essen et al., 2014). Transcription factors such as ID2, ZEB1 and TWIST1 are involved in UM growth and invasiveness (Chen et al., 2017), expression of PD-1 in UM cells avoids immune destruction by suppressing T cell, facilitating tumor dissemination, and resistance to chemotherapies (Komatsubara and Carvajal, 2017).
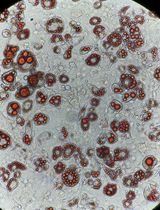
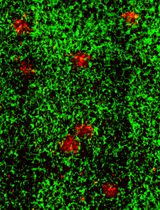
Appropriate animal models for UM are critical in understanding molecular mechanisms and evaluating therapeutic effectiveness. Mice are most commonly utilized for tumor models. No spontaneous UM was found in wild-type mice (Stei et al., 2016). Mutation in GNAQ gene could generate choroidal melanoma in mice, but the tumor exclusively metastasizes to the lung (Huang et al., 2015). To date, no genetic animal model mimicking the aforementioned biological and molecular features of human UM has been generated (Stei et al., 2016). By contrast, mouse intraocular xenograft tumor is a widely accepted UM animal model with an effective formation of primary ocular tumors and potential to metastasize to the liver. This article details a protocol to xenograft human UM cells in the vitreous cavity of nude mice, which develops primary tumors in the eye and metastases in the liver in a relatively short period of time.
Materials and Reagents
- 100-mm culture plate (Corning, catalog number: 430293 )
- 15-ml tube (Corning, catalog number: 430052 )
- 30 G blunt needle (BD, catalog number: 305106 )
- Cotton swabs (VWR, catalog number: 89031-270 )
- Glass coverslips (Fisher Scientific, catalog number: 12-550-15 )
- Diapers (VWR, catalog number: 82020-845 )
- Surgeon masks (VWR, catalog number: 10843-149)
Manufacturer: KCWW, Kimberly-Clark, catalog number: 47500 . - Gloves (VWR, catalog number: 82026-426 )
- Head cover (3M, catalog number: S-133S-5 )
- 1-ml syringe (BD, catalog number: 309628 )
- 27 G needle (BD, catalog number: 305109 )
- 200-μl tips (USA Scientific, catalog number: 1111-1700 )
- Athymic nude mice (THE JACKSON LABORATORY, catalog number: 002019 )
- Human UM cell line OCM1 (provided by Dr. Klara Valyi-Nagy in the University of Illinois at Chicago)
- 0.25% trypsin (Mediatech, catalog number: 20-053-Cl )
- Phosphate-buffered saline (PBS) (Mediatech, catalog number: 21-040-CV )
- Mydriatic eye drops are the mixture of Phenylephrine Hydrochloride Ophthalmic Solution (Paragon, NDC 42702-102-15) and Tropicamide Ophthalmic Solution (Bausch & Lomb, NDC 24208-585-64) at the ratio of 1:1
- Hypromellose ophthalmic demulcent solution (Akorn, NDC 17478-064-12)
- GONAK Lubricant (2.5% Hypromellose ophthalmic demulcent solution) (Akorn, NDC 17478-064-12)
- Povidone-Iodine solution (Dynarex, NDC 67777-100-03)
- Ketamine hydrochloride (Hospira, NDC 0409-2053-10)
- Xylazine sterile solution (Akorn, AnaSed®, NDC 59399-110-20)
- Neomycin and polymyxin B sulfates and dexamethasone ophthalmic ointment (Fera, NDC 48102-003-35)
- Meloxicam (Henry Schein, NDC 11695-6925-2)
- 10% neutral buffered formalin (Sigma-Aldrich, catalog number: F5554-4L )
- Ethanol (Sigma-Aldrich, catalog number: 459836-1L )
- Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, catalog number: 10-013-CV )
- Fetal bovine serum (FBS) (GE Healthcare, HycloneTM, catalog number: SH30070.03HI )
- Penicillin-streptomycin solution (Mediatech, catalog number: 30-002-Cl )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D5879-500ML )
- Cell culture medium (see Recipes)
- Cell cryopreservation medium (see Recipes)
Equipment
- Cell culture incubator (Thermo Fisher Scientific, Thermo ScientificTM, model: Series 8000 Water-Jacketed , catalog number: 3423)
- Bench top centrifuge (Fisher Scientific, model: accuSpin 24CTM )
- -80 °C freezer (Thermo Fisher Scientific, Thermo ScientificTM, model: TSUTM Series , catalog number: TSU400A-EA)
- 5-μl syringe (Hamilton, catalog number: 87900 )
- 50-ml beaker (VWR, catalog number: 10754-946 )
- Forceps (VWR, catalog number: 82027-404 )
- Scissors (VWR, catalog number: 89259-982 )
- 200-µl pipet (Eppendorf, catalog number: 3123000055 )
- Liquid nitrogen tank (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: CY50945 )
- Surgical microscope (Olympus, model: SZ61/51 )
- Warm pad (Pristech Products, catalog number: 20414 )
- Autoclave (SOMA Technology, model: Steris Amsco Century V116 )
- -20 °C freezer (Kelvinator Commercial, model: KCBM180FQY )
- Sterile hood (Labconco, model: Class II, Type B2 (Total Exhaust) )
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Chen, Y., Liu, X., Gao, L. and Liu, Y. (2017). Xenograft Mouse Model of Human Uveal Melanoma. Bio-protocol 7(21): e2594. DOI: 10.21769/BioProtoc.2594.
分类
癌症生物学 > 通用技术 > 动物模型
细胞生物学 > 细胞移植 > 异种移植
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link