- EN - English
- CN - 中文
Determination of Local pH Differences within Living Salmonella Cells by High-resolution pH Imaging Based on pH-sensitive GFP Derivative, pHluorin(M153R)
采用基于pH敏感性GFP衍生物pHluorin(M153R)的高分辨率pH成像测定活体沙门氏菌细胞中局部pH值的差异
发布: 2017年09月05日第7卷第17期 DOI: 10.21769/BioProtoc.2529 浏览次数: 7674
评审: Dennis NürnbergEunsook ParkRon Saar-Dover
Abstract
The bacterial flagellar type III protein export apparatus is composed of a transmembrane export gate complex and a cytoplasmic ATPase complex. The export apparatus requires ATP hydrolysis and the proton motive force across the cytoplasmic membrane to unfold and transport flagellar component proteins for the construction of the bacterial flagellum (Minamino, 2014). The export apparatus is a proton/protein antiporter that couples the proton flow with protein transport through the gate complex (Minamino et al., 2011). A transmembrane export gate protein, FlhA, acts as an energy transducer along with the cytoplasmic ATPase complex (Minamino et al., 2016). To directly measure the proton flow through the FlhA channel that is coupled with the flagellar protein export, we have developed an in vivo pH imaging system with high spatial and pH resolution (Morimoto et al., 2016). Here, we describe how we measure the local pH near the export apparatus in living Salmonella cells (Morimoto et al., 2016). Our approach can be applied to a wide range of living cells. Because local pH is one of the most important parameters to monitor cellular activities of living cells, our protocol would be widely used for diverse areas of life sciences.
Keywords: Intracellular pH (细胞内pH)Background
The proton channel activities of transmembrane proton channel complexes have been detected as reduction in cytoplasmic pH of bacterial cells (Morimoto et al., 2010; Che et al., 2014; Furukawa et al., 2017). However, to measure the proton channel activity of membrane complexes in living cells in detail, precise measurements of the local cytoplasmic pH are required. A derivative of the green fluorescence protein (GFP), pHluorin, with excitation at wavelengths of 410 and 470 nm and emission at 508 nm is a useful probe to measure the cytoplasmic pH in living cells (Miesenböck et al., 1998). This probe enables us to measure the intracellular pH precisely and quantitatively because the fluorescent intensity ratio of the two excitation wavelengths R410/470 shows a remarkable pH dependence. pHluorin can be fused genetically to a target protein to monitor the local pH around the protein. However, proteolytic cleavage often removes pHluorin from its fusion protein, not only causing a poor signal-to-noise ratio but also leading to the wrong interpretations of the data. We have found that the mutation M153R in pHluorin significantly stabilizes its fusion products while retaining the marked pH dependence of the 410/470 nm excitation ratio of the fluorescence intensity (Morimoto et al., 2011). We therefore used pHluorin(M153R) to develop a method to measure the local cytoplasmic pH in living bacterial cells in a highly quantitative and precise manner (Morimoto et al., 2016).
Materials and Reagents
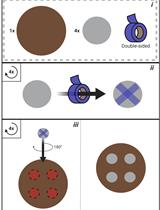
- 1.5 ml microcentrifuge tubes
- Glass test tubes (IWAKI, catalog number: A-18P )
- Double-sided tape (NICHIBAN, catalog number: NW-5 )
- 24 x 32 mm coverslips (thickness: 0.12-0.17 mm) (Matsunami Glass, catalog number: C024321 )
- 18 x 18 mm coverslips (thickness: 0.12-0.17 mm) (Matsunami Glass, catalog number: C018181 )
- Pipette tips
- Filter paper (Toyo Roshi Kaisha, ADVANTEC, catalog number: 00011090 )
- Salmonella YVM1004 strain (pHluorin(M153R)-fliG) (Morimoto et al., 2011)
- Salmonella YVM1049 strain (∆fliH-fliI flhB(P28T) pHluorin(M153R)-fliG) (Morimoto et al., 2016)
- Salmonella SJW1103 strain (wild type for motility and chemotaxis) (Yamaguchi et al., 1984)
- Salmonella SJW1368 strain (∆(cheW-flhD); flagellar master operon mutant) (Ohnishi et al., 1994)
- pYVM008 (pTrc99A/pHluorin(M153R)-FliG) (Morimoto et al., 2016)
- Purified pHluorin(M153R)-FliG-His6 protein in PBS
Note: The pHluorin(M153R)-FliG-His protein is purified by nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography from the soluble fractions of E. coli BL21(DE3) cells overexpressing pHluorin(M153R)-FliG-His as described by Minamino et al. (2000). - Ampicillin sodium salt (Wako Pure Chemical Industries, catalog number: 014-23302 )
- Gramicidin (Thermo Fisher Scientific, catalog number: G6888 )
- Potassium benzoate (Wako Pure Chemical Industries, catalog number: 164-19342 )
- Bacto tryptone (BD, BactoTM, catalog number: 211705 )
- Yeast extract (BD, BactoTM, catalog number: 212750 )
- Sodium chloride (NaCl) (Wako Pure Chemical Industries, catalog number: 192-13925 )
- Bacto agar (BD, BactoTM, catalog number: 214010 )
- Potassium dihydrogen phosphate (KH2PO4) (Wako Pure Chemical Industries, catalog number: 164-22635 )
- Dipotassium hydrogen phosphate (K2HPO4) (Wako Pure Chemical Industries, catalog number: 164-04295 )
- Ethylenediamine-N,N,N’,N’-tetraacetic acid disodium salt dihydrate (2NA) (EDTA) (Dojindo, catalog number: N001 )
- LB medium (see Recipes)
- TB medium (see Recipes)
- LB agar plate (see Recipes)
- Motility buffer (see Recipes)
Equipment
- pH meter (Beckman Coulter, model: Φ34 )
- Shaking incubator (30 °C, at 200 rpm) (TAITEC, model: BR-40LF )
- Spectrophotometer (able to measure OD600) (Shimadzu, model: UV-1800 )
- Centrifuge (able to hold 1.5 ml tubes, spin at 6,000 x g) (TOMY SEIKO, model: MX-305 )
- Single channel pipettes (1,000 µl, 100 µl) (PIPETMAN® Classic; Gilson, models: P1000 and P100 )
- Inverted fluorescence microscope (Olympus, model: IX71 ) (see Figure 1)
- 150x oil immersion objective lens (Olympus, model: UApo150XOTIRFM , NA1.45)
- Electron-multiplying charge-coupled device (EMCCD) camera (Hamamatsu Photonics, model: C9100-02 )
- Excitation filter (Omega Optical, model: 400AF30 )
- Excitation filter (Olympus, model: BP470-490 )
- Emission filter (Omega Optical, model: 520DF40 )
- High-speed wavelength switcher (Sutter Instrument, model: Lambda DG-4 )
- Dichroic mirror (Semrock, model: FF510-Di01-25x36 )
Software
- MetaMorph (Molecular Devices)
- ImageJ (National Institutes of Health, https://imagej.nih.gov/ij/)
- IGOR Pro (WaveMetrics)
- Microsoft Excel (Microsoft)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Morimoto, Y. V., Kami-ike, N., Namba, K. and Minamino, T. (2017). Determination of Local pH Differences within Living Salmonella Cells by High-resolution pH Imaging Based on pH-sensitive GFP Derivative, pHluorin(M153R). Bio-protocol 7(17): e2529. DOI: 10.21769/BioProtoc.2529.
分类
微生物学 > 微生物细胞生物学 > 细胞成像
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link