- EN - English
- CN - 中文
Assaying Glycogen and Trehalose in Yeast
酵母中糖原和海藻糖的测定
发布: 2017年07月05日第7卷第13期 DOI: 10.21769/BioProtoc.2371 浏览次数: 10455
评审: Gal HaimovichAnonymous reviewer(s)
Abstract
Organisms store carbohydrates in several forms. In yeast, carbohydrates are stored in glycogen (a multi-branched polysaccharide) and in trehalose (a disaccharide). As in other organisms, the amount of stored carbohydrate varies dramatically with physiological state, and accordingly, an assay of stored carbohydrate can help reveal physiological state. Here, we describe relatively easy and streamlined assays for glycogen and trehalose in yeast that can be applied either to a few samples, or in a moderately high-throughput fashion (dozens to hundreds of samples).
Keywords: Glycogen (糖原)Background
Glycogen and trehalose are the two storage carbohydrates of yeast and many other organisms. In yeast, both these storage carbohydrates accumulate when the medium starts to be depleted and the rate of cell growth decreases. Methods for assaying storage carbohydrates in yeast date back at least to 1956 (Trevelyan and Harrison, 1956a and 1956b), and have been updated many times since (e.g., [Becker, 1978; Quain, 1981; Schulze et al., 1995; Parrou and Francois, 1997; Plata et al., 2013], among others). There are three basic steps in assaying these two storage carbohydrates: first, lysing or permeabilizing the cells; second, freeing glucose from the glycogen or trehalose; and third, assaying the resulting glucose.
Cells can be lysed mechanically (Schulze et al., 1995), but this is inevitably somewhat tedious and time-consuming, and tends to require larger numbers of cells. Cells can be permeabilized by alkali, but glycogen forms large, multi-branched granules, and can be difficult to extract, and so some protocols use both an alkali and an acid extraction (Trevelyan and Harrison, 1956a and 1956b; Quain, 1981). However, alkali treatment alone extracts the vast majority of the glycogen (and probably all of the trehalose) (Becker, 1978; Quain, 1981; Parrou and Francois, 1997); and it may allow enzymes such as amyloglucosidase access to the interior of the permeabilized cell, where it can liberate glucose from any residual glycogen, and alkali extraction alone is much easier than a dual alkali/acid extraction. Therefore, like Becker, and Parrou and Francois, we use only an alkali extraction. However, it is possible that this may fail to assay a relatively small amount of acid-extractable glycogen (Quain, 1981).
In older assays (e.g., [Trevelyan and Harrison, 1956a and 1956b]), glucose was released and/or assayed by purely chemical methods. However, these were relatively non-specific, and also assayed glucose present in other molecules, such as cell wall glucans. Therefore more modern methods use enzymes to liberate glucose from specific polysaccharides; e.g., amyloglucosidases are used to liberate glucose from glycogen (Becker, 1978), and trehalases are used to liberate glucose from trehalose (Parrou and Francois, 1997). A challenge to these methods is that some enzymes are contaminated with other activities. For instance, Parrou and Francois found that some amyloglucosidases were contaminated with trehalases. Therefore either purer enzymes need to be used, or less pure enzymes need to be used under conditions that inhibit the unwanted activities. Here, like Parrou and Francois, we use Aspergillus niger α-amyloglucosidase, which may also contain a trehalase activity (Parrou and Francois, 1997), depending on the specific preparation of enzyme, but we use it at high temperature (55 °C to 57 °C), approximately the optimum temperature for this enzyme, where the trehalase is inactive (Parrou and Francois, 1997).
Finally, the enzymatically-released glucose must be assayed. There are many well-developed assays for glucose. We use the glucose oxidase/peroxidase/o-dianisidine reagent of the Sigma-Aldrich glucose oxidase kit, which produces oxidized o-dianisidine, which has a pink/purple color, easily assayed by absorbance at 540 nm.
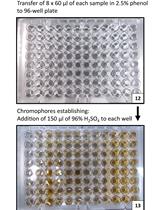
Our procedure is adapted from that of Parrou and Francois (1997). However, at most steps, we use smaller volumes of reagents, which make the assay easier in some respects. The small volumes allow us to adapt the procedure to 96-well microtitre dishes, which allows the assay to become moderately high-throughput. We give two procedures, one for 2 ml screw-capped tubes, and one for 96-well microtitre dishes.
Materials and Reagents
- Protective eye wear/safety glasses/face shield
- Pipette tips
- 2 ml screw cap tubes with o-ring (e.g., SARSTEDT, catalog number: 72.694.406 or 72.694.217 )
- Microplate sealing tape (e.g., Corning aluminum tape, Corning, catalog number: 6570 )
- QuickSeal Foil PCR Self Adhesive Seal (Biosero)
Or 4titude PCR Foil Seal (4titude, catalog number: 4ti-0550 )
Or Peelable heat-sealing foil seals and a heat sealer - For 96 well microtitre plate assay
a.Polypropylene, round-bottom 96-well plates, 360 microlitre capacity (e.g., Corning, catalog number: 3359 )
b.Polystyrene, flat-bottom 96-well plates (for plate reader) (e.g., Corning, catalog numbers: 3370 and 3915 ) - Yeast cells
Note: This protocol has been developed for S. cerevisiae. It has not been tried with other species of yeast, but should work. - Milli-Q or double-distilled water
- Glucose assay kit (Sigma-Aldrich, catalog number: GAGO-20 )
- Sulphuric acid
- Glacial acetic acid
- NaAcetate trihydrate*
- Aspergillus niger α-amyloglucosidase (Biochemika, ~70 U/mg) (Sigma-Aldrich, catalog number: 10115 )
Alternatively: 120 U/mg, may be higher purity (Sigma-Aldrich, catalog number: 10113 ). - Porcine trehalase (about 2.3 U/ml) (Sigma-Aldrich, catalog number: T8778 )
- Concentrated H2SO4 (sulfuric acid)*
- Sodium carbonate anhydrous (Na2CO3)*
- 1 M acetic acid (see Recipes)*
- 0.2 M NaAcetate, pH 5.2 (see Recipes)
- 0.2 M NaAcetate, ~pH 8 (see Recipes)
- For 96 well microtitre plate assay
- Concentrated amyloglucosidase buffer (see Recipes)
- Concentrated trehalase buffer (see Recipes)
- Trehalase dilution buffer (0.1 M NaAcetate, pH 5.7) (see Recipes)
- 9 N H2SO4 (see Recipes)
- 0.25 M Na2CO3 (see Recipes)
Note: *Reagents from any qualified company are suitable for this experiment.
Equipment
- Roller or shaker for growing yeast
- Adjustable micropipettes, volumes from 2 to 500 μl
- Spectrophotometer and cuvettes
- Centrifuge (room temperature or chilled) for volumes of 5 to 15 ml
- Microcentrifuge for 1.5 and 2 ml tubes
- Vortex mixer
- pH meter
- Water bath (95 °C)
- Water bath or air incubator, 57 °C and 37 °C
- Glass pipet
- Fume hood
- For 96-well plate assay
- Centrifuge and adaptors for microtitre plates
- Multichannel pipettes
- Plate reader
Note: All those items can be ordered from any qualified company.
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Chen, Y. and Futcher, B. (2017). Assaying Glycogen and Trehalose in Yeast. Bio-protocol 7(13): e2371. DOI: 10.21769/BioProtoc.2371.
分类
微生物学 > 微生物新陈代谢 > 糖类
生物化学 > 糖类 > 糖原
生物化学 > 糖类 > 二糖
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link