- EN - English
- CN - 中文
Thermostability Measurement of an α-Glucosidase Using a Classical Activity-based Assay and a Novel Thermofluor Method
采用经典基于活性的测定法和新型Thermofluor法测定α-葡萄糖苷酶的热稳定性
发布: 2017年06月20日第7卷第12期 DOI: 10.21769/BioProtoc.2349 浏览次数: 10582
评审: Yanjie LiAyelign M. AdalAnonymous reviewer(s)
Abstract
α-glucosidases (including maltases and isomaltases) are enzymes which release glucose from a set of α-glucosidic substrates. Their catalytic activity, substrate specificity and thermostability can be assayed using this trait. Thermostability of proteins can also be determined using a high-throughput differential scanning fluorometry method, also named Thermofluor. We have shown that Thermofluor can also be applied to predict binding of substrates and inhibitors to a yeast α-glucosidase. The methods described here in detail were used in Viigand et al., 2016.
Keywords: Maltase (麦芽糖酶)Background
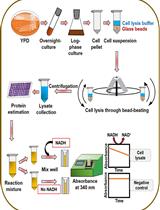
Maltases (EC 3.2.1.20) and isomaltases (EC 3.2.1.10) are α-glucosidases belonging to family 13 of glycoside hydrolases according to the CAZy classification (Lombard et al., 2014). Maltase MAL1 of a methylotrophic yeast Ogataea polymorpha is nonselective–it hydrolyses maltose- and isomaltose-like α-glucosidic sugars producing D-glucose as one of the reaction products. Thus, activity of maltase on its substrates can be determined according to glucose release. The Glucose liquicolor-aided method described in this work allows rapid and convenient assay of the activity, substrate specificity and thermostability of the maltase. Importantly, this activity-based method can be adapted to other enzymes that produce glucose as a reaction product. A high-throughput Thermofluor method is mostly used in protein crystallography to measure (thermal) stability of the protein (Boivin et al., 2013; Ericsson et al., 2006). We used Thermofluor 1) to evaluate thermostability of the maltase protein and 2) to study its substrate specificity (Viigand et al., 2016). Substrate specificity assay of glycoside hydrolases and other sugar-acting enzymes using Thermofluor is cost-efficient–it requires very low amounts of the protein as well as ligand sugars that can be very expensive. Regarding substrates of α-glucosidases, one gram of isomaltose from Sigma-Aldrich costs almost 1,000 euros, 10 milligrams of nigerose 143 euros and 1 mg of kojibiose almost 200 euros.
Materials and Reagents
- For both methods
- 1.5 ml microtubes (Corning, Axygen®, catalog number: MCT-150-C )
- 0.2 μm cellulose acetate membrane filter (Sartorius, catalog number: 11107-47-N )
- Sucrose (Sigma-Aldrich, catalog number: 16104 )
- MilliQ quality water (MQ)
- Crushed ice
- In-house laboratory purified C-terminally His-tagged maltase MAL1 (from Ogataea polymorpha) and its inactive mutant protein (Asp199Ala) overexpressed in Escherichia coli (prepared as in Viigand et al., 2016)
- 1.5 ml microtubes (Corning, Axygen®, catalog number: MCT-150-C )
- For classical activity assay
- Special PS (polystyrene) micro photometer cuvette, 2 ml (LP ITALIANA, catalog number: 112117 )
- Glucose liquicolor kit (GOD-PAP Method, Enzymatic Colorimetric Test for Glucose) (Human, catalog number: 10260 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: 795488 )
- Potassium phosphate dibasic (K2HPO4) (Sigma-Aldrich, catalog number: RES20765 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E9884 )
- Tris base (Roche Molecular Systems, catalog number: 10708976001 )
- Hydrochloric acid 37% (HCl) (AppliChem, catalog number: A0659 )
- 1 M Tris-HCl buffer (pH 8.3) (see Recipes)
- Maltase buffer (see Recipes)
- Special PS (polystyrene) micro photometer cuvette, 2 ml (LP ITALIANA, catalog number: 112117 )
- For Thermofluor method
- LightCycler® 480 Multiwell Plate 96 (white) with sealing foils (Roche Molecular Systems, catalog number: 04729692001 )
- 5,000x SYPRO Orange Protein Gel Stain (Sigma-Aldrich, catalog number: S5692 )
- HEPES buffer (Sigma-Aldrich, catalog number: H3375 )
- Sodium hydroxide (NaOH) (AppliChem, catalog number: 131687.1211 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 31434-M )
- 0.5 M HEPES buffer (pH 7.0) (see Recipes)
- 4x Thermofluor buffer (see Recipes)
- LightCycler® 480 Multiwell Plate 96 (white) with sealing foils (Roche Molecular Systems, catalog number: 04729692001 )
Equipment
- Pharmaceutical balance PS2100/C/2 (RADWAG Balances and Scales, model: PS 2100/C/2 )
- JENWAY pH meter model 3510 (Cole-Parmer Instrument, model: Jenway 3510 )
- Pipettes (FisherbrandTM Elite Pipette Kit) (Fisher Scientific, catalog number: 14-388-100 )
- Refrigerator-freezer (Electrolux, model: EN2900AOW )
- Ultrospec 3100 pro UV/Visible spectrophotometer (GE Healthcare, Amersham Biosciences, model: Ultrospec 3100 pro , catalog number: 80-2112-37)
- ThermoBlock TDB-120 (Biosan, model: TDB-120 )
- Digital timer/clock (Fisher Scientific, catalog number: S01619 )
- Plate sealer spatula for microtiter plates (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 5701 )
- LightCycler® 480 Instrument II (Roche Molecular Systems, model: LightCycler® 480 Instrument II , catalog number: 05015278001)
- Refrigerated centrifuge 4K-15 (Sigma Laborzentrifugen, model: 4K-15 , catalog number: 10742) with the swing-out rotor for 4 buckets (Sigma Laborzentrifugen, catalog number: 11150 ) and bucket, aluminium, for microtiter plates (Sigma Laborzentrifugen, catalog number: 13220 )
Note: A classical thermostability assay of the maltase is based on determination of residual catalytic activity of the enzyme after its incubation at various temperatures. Therefore, we first give the protocol for the measurement of maltase activity.
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ernits, K., Viigand, K., Visnapuu, T., Põšnograjeva, K. and Alamäe, T. (2017). Thermostability Measurement of an α-Glucosidase Using a Classical Activity-based Assay and a Novel Thermofluor Method. Bio-protocol 7(12): e2349. DOI: 10.21769/BioProtoc.2349.
分类
生物化学 > 蛋白质 > 荧光
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link