- EN - English
- CN - 中文
Functional ex-vivo Imaging of Arterial Cellular Recruitment and Lipid Extravasation
动脉细胞招募和脂质外渗的功能性离体成像
发布: 2017年06月20日第7卷第12期 DOI: 10.21769/BioProtoc.2344 浏览次数: 11285
评审: Gal HaimovichVasiliki KoliarakiAnonymous reviewer(s)
Abstract
The main purpose of this sophisticated and highly versatile method is to visualize and quantify structural vessel wall properties, cellular recruitment, and lipid/dextran extravasation under physiological conditions in living arteries. This will be of interest for a broad range of researchers within the field of inflammation, hypertension, atherosclerosis, and even the pharmaceutical industry. Currently, many researchers are using in vitro techniques to evaluate cellular recruitment, like transwell or flow chamber systems with cultured cells, with unclear physiological comparability. The here introduced method describes in detail the use of a sophisticated and flexible method to study arterial wall properties and leukocyte recruitment in fresh and viable murine carotid arteries ex vivo under arterial flow conditions. This model mimics the in vivo situation and allows the use of cells and arteries isolated from two different donors (for example, wildtype vs. specific knockouts) to be combined into one experiment,thereby providing information on both leukocyte and/or endothelial cell properties of both donors. As such, this model can be considered an alternative for the complicated and invasive in vivo studies, such as parabiotic experiments.
Keywords: Imaging (成像)Background
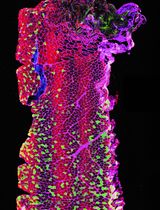
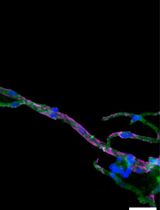
The core of the method is the application of two-photon laser scanning microscopy (TPLSM) to visualize an ex vivo carotid artery which is mounted in an arteriograph chamber, which has been shown to mimic physiological conditions as present in the in vivo situation (Megens et al., 2007). Fresh arteries, in our case murine carotid arteries but the method is also applicable for other blood vessels of comparable size including human vessels (Bloksgaard et al., 2015), are carefully extracted and mounted in the arteriograph chamber on two glass micropipettes using thin threads. The chamber should provide sufficient space to access the artery with the microscope objective (preferably water dipping or immersion objective with a working distance of > 1 mm). After applying luminal pressure (80 mmHg) and the subsequent correction of the shortening of the length due to isolation (Megens et al., 2007), a variety of fluorescently labeled cells and/or vessel wall components of interest can be perfused into the vessel either under flow or under static conditions. This enables the user to A) count the number of adherent leukocyte subsets, B) determine molecular extravasation (dextrans, lipids) into the arterial wall, C) visualize vascular properties and structures using fluorescently conjugated (specific) antibodies or intrinsic fluorescence signals derived from extracellular matrix components, D) combine the previously described targets.
The general basis of this method was described in 2007 (Megens et al., 2007). Since then this method has been used in several scientific publications, for example to show cell recruitment under control and inflammatory conditions (Schmitt et al., 2014), chemokine presence (Soehnlein et al., 2011), detection of smooth muscle cells (Subramanian et al., 2010; Spronck et al., 2016) or proliferating endothelial cells (Schober et al., 2014), endothelial protein depositions (Ortega-Gomez et al., 2016), adhering platelets (Karshovska et al., 2015), atherosclerotic lesions in the bifurcation (Megens et al., 2007 and 2008; Weber et al., 2011), visualization of the endothelial glycocalyx (Reitsma et al., 2011), or evaluation of extracellular matrix markers (Boerboom et al., 2007; Megens et al., 2007).
TPLSM imaging can be performed prior-, during-, and/or post-perfusion. The settings of the microscope system strongly depend on the available microscope. We utilize a modern Leica SP5II MP system with a 20x WD objective and a Ti:Sa pulsed laser which allows 4 channel imaging at video rate. This is however not a requirement for application of this method as older, less well equipped TPLSM systems also suffice.
In recent years, we have further advanced the method, making it applicable to investigate recruitment of specific cell-types to the viable carotid artery (Döring et al., 2014; Schmitt et al., 2014; Karshovska et al., 2015). Not only does this method enable the user to specifically and simultaneously investigate recruitment of various cell types like monocytes, neutrophils or T-cells to highly physiological endothelium by differential fluorescent labeling (using cell trackers or equivalent), it also allows us to combine specific arteries and cells isolated (blood, bone marrow) from different (wildtype vs. knockout) mouse subsets. As a result, a system is created that can define whether effects on recruitment are mediated by the vascular and/or haematopoietic deficiency. Besides the functional readout of cell recruitment, the method further enables simultaneous or subsequent labelling and subcellular resolution imaging of vascular structures and presence of compounds in the vessel wall. As a result, altered adhesion may directly be linked to the presence or absence of specific targets.
By simultaneous application of various fluorescently labeled leukocyte subsets, the experimental conditions are equal for each cell-type, thereby limiting the experimental variation due to for example flow pattern differences (data may be presented in absolute numbers or ratios). The latter also limits the number of experiments required and, in combination with a reduced number of necessary experimental animals because inflammatory cells and arteries can be isolated from the same animals, ultimately the method reduces the number of required animals.
In addition to the cell recruitment assay we have developed an application using fluorescently labeled low-density lipoprotein particles or dextrans to visualize and quantify lipid or dextran extravasation in viable (diseased) arteries. Lastly, unlike in vivo imaging of large arteries, this ex vivo model does not suffer from unwanted motions of the arterial wall as is the case in the in vivo situation, thereby allowing imaging with subcellular resolution. Moreover, stimuli or specific dyes may be applied at any given time during the experiment giving the researcher full flexibility to tailor the methodology and achieve the required goals.
Materials and Reagents
- Isolation of carotid arteries
- Cell suspension
- 50 ml tubes (3 x) (SARSTEDT, catalog number: 62.547.254 )
- Needle 27 G x 1.5 (Grey) (BD, catalog number: 301629 )
- Syringe 10 ml (1 x) (BD, DiscarditTM catalog number: 309110 )
- Cell strainer 50 µm (Sysmex, CellTrics®, catalog number: 25004-0042-2317 )
- Hanks balanced saline solution (HBSS) with CaCl2 and MgCl2 (Thermo Fisher Scientific, catalog number: 1402550 ), pH 7.4
- Fluorescent cell markers: cell tracker green (Thermo Fisher Scientific, InvitrogenTM, catalog number: C7025 ) and Red (Thermo Fisher Scientific, InvitrogenTM, catalog number: C34565 )
- Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: A9434 )
- Potassium bicarbonate (KHCO3) (Sigma-Aldrich, catalog number: 60339 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E6758 )
- Lysis buffer (see Recipes)
- 50 ml tubes (3 x) (SARSTEDT, catalog number: 62.547.254 )
- Mounting of artery
- Glass etching material (NH4HF2 or NH4F·HF) (Carglass, catalog number: ZB10 EI0003 )
- Needles 14 G x 80 mm, shortened and blunted to 40 mm and 50 mm (Braun Sterican 14 G x 80 mm) (B. Braun Medical, catalog number: 4665473 )
- Nylon thread Ø ~20 µm, for tying of blood vessels (Living Systems Instruments, catalog number: THR-G )
- Three-way tab (2 x) (B. Braun Medical, catalog number: 4095111 )
- Syringe 1 ml (BD, PlastipakTM, catalog number: 300026 )
- Silicone tubing 3 x 1 mm (Carl Roth, catalog number: 9556.1 ) cut to 0.5 m length
- Hanks balanced saline solution (HBSS) with CaCl2 and MgCl2 (Thermo Fisher Scientific, catalog number: 1402550 ), pH 7.4
- Glass etching material (NH4HF2 or NH4F·HF) (Carglass, catalog number: ZB10 EI0003 )
- Molecular extravasation
- Silicone tubing 3 x 1 mm cut to 1.0 m length (Carl Roth, catalog number: 9556.1 )
- Needle 20 G x 1.5 (yellow) (BD, catalog number: 301300 )
- Safe lock tubes 1.5 ml (3 x) (Eppendorf, catalog number: 0030120086 )
- Syringes 1 ml (2 x) (BD, PlastipakTM, catalog number: 300026 )
- Three-way tab (B. Braun Medical, catalog number: 4095111 )
- Fixation tape Durapore 1.25 cm (3M, catalog number: 1538-0 )
- Human Dil-LDL (Kalen Biomedical, catalog number: 770230-9 )
- Hanks balanced saline solution (HBSS) with CaCl2 and MgCl2 (Thermo Fisher Scientific, catalog number: 1402550 ), pH 7.4
- Directly conjugated anti-CD31/eFluor450 (PECAM: Thermo Fisher Scientific, eBioscienceTM, catalog number: 48-0311-82 )
Alternatives: Directly conjugated anti-CD54/A488 (ICAM: BioLegend, catalog number: 116112 ) or anti-CD106/A594 (VCAM: BioLegend, catalog number: 105724 )
- Silicone tubing 3 x 1 mm cut to 1.0 m length (Carl Roth, catalog number: 9556.1 )
- Flow assay
- Silicone tubing 3 x 1 mm cut to 1.5 m and 1.0 m length (Carl Roth, catalog number: 9556.1 )
- Needle 20 G x 1.5 (yellow) (BD, catalog number: 301300 )
- Syringes 1 ml (1 x) (see Reagent C5) and 10 ml (2 x) (BD, DiscarditTM, catalog number: 309110 )
- Three-way Tab (2 x) (B. Braun Medical, catalog number: 4095111 )
- 50 ml tubes (2 x) (SARSTEDT, catalog number: 62.547.254 )
- Fixation tape Durapore 1.25 cm (3M, catalog number: 1538-0 )
- Hanks balanced saline solution (HBSS) with CaCl2 and MgCl2 (Thermo Fisher Scientific, catalog number: 1402550 ), pH 7.4
- Silicone tubing 3 x 1 mm cut to 1.5 m and 1.0 m length (Carl Roth, catalog number: 9556.1 )
Equipment
- Isolation of carotid arteries
- Pen
- FST student spring scissors (Fine Science Tools, catalog number: 91500-09 )
- Dumont forceps (2 x) (Fine Science Tools, catalog number: 91150-20 )
- Scissors (Fine Science Tools, catalog number: 14084-08 )
- Stereomicroscope Leica S8 Apo (LED light source and 0.63x objective) (Leica Microsystems, model: Leica S8 Apo )
- Pen
- Cell suspension
- Timer
- Pen (see Equipment A1)
- Scissors (Fine Science Tools, catalog number: 91401-12 )
- 1 ml pipette (Eppendorf, catalog number: 3120000062 )
- Centrifuge (Eppendorf, model: 5430 )
- Cell counting chamber (Neubauer)
- Cell culture microscope (Leica DMi1 with phase contrast and 10x NA0.3 objective) (Leica Microsystems, model: Leica DMi1 )
- Flow cytometer (BD, model: BD FACSCANTO SYSTEM )
- Timer
- Mounting of artery
- Arteriograph chamber (2 x, IDEE©, Maastricht University, the Netherlands)
- Glass pipettes 1.5 x 0.86 mm (2 x, Harvard Apparatus, catalog number: 30-0057 )
- Pipette puller (NARISHIGE, catalog number: PP-830 )
- Pipette tip grinder (IDEE©, Maastricht University, the Netherlands)
Alternative: Glass pipettes readymade (Living Systems Instruments, catalog number: GCP-300-325 ) - Silicone kit, 73 clear (Farnell, catalog number: 101705 )
- Sphygmomanometer (Riester, Big Ben®, catalog number: 1453-100 ) adapted with a 500 ml air chamber (Schott) and Luer-connectors to fit 1 x 3 mm silicone tubing (IDEE©, Maastricht University, the Netherlands)
- Luer-coupling adapter female (1x) (Carl Roth, catalog number: CT62.1 )
- Stereomicroscope Leica S8 Apo (with LED light source and 0.63x objective) (Leica Microsystems, model: Leica S8 Apo)
- Arteriograph chamber (2 x, IDEE©, Maastricht University, the Netherlands)
- Microscope
- Commercially available Leica SP5IIMP laser scanning microscope system based on a DM6000FS microscope stand (for more info we refer to the manufacturer’s website:
https://www.leica-microsystems.com/products/confocal-microscopes/details/product/leica-tcs-sp5-ii/downloads/)
Alternative: Any functional TPLSM enabling spectral specificity in ≥ 2 detectors and a water dipping objective with sufficient working distance (≥ 1 mm) can be used in combination with the here described assays. - Spectra physics MaiTai DeepSee Ti:Sa laser source
- Leica Hybrid detectors (4 x)
- Luigs and Neumann SM7 motorized microscope stage
- Leica 20x NA1.00WD objective
- Ludin Climate control /laser safety box
- Commercially available Leica SP5IIMP laser scanning microscope system based on a DM6000FS microscope stand (for more info we refer to the manufacturer’s website:
- Molecular extravasation
- Flow assay
- Luer-coupling adapters: male (2 x) (Carl Roth, catalog number: CT58.1 ) and female (1 x) (Carl Roth, catalog number: CT62.1 )
- Syringe infusion pump (Harvard Apparatus pump11 elite) (Harvard Apparatus, catalog number: 70-4504 )
- Column stand with clamp (unknown brand, length 100 cm, footprint 15 x 25 cm)
- Pc with excel and/or paper for cell count
- Luer-coupling adapters: male (2 x) (Carl Roth, catalog number: CT58.1 ) and female (1 x) (Carl Roth, catalog number: CT62.1 )
Software
- Leica LAS AF 2.6 acquisition software
- Leica LAS X 3.11 image processing software (including 3D analyses package); offline
Note: Any processing software package that allows the user to handle multidimensional data can be used.
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
van der Vorst, E. P., Maas, S. L., Ortega-Gomez, A., Hameleers, J. M., Bianchini, M., Asare, Y., Soehnlein, O., Döring, Y., Weber, C. and Megens, R. T. (2017). Functional ex-vivo Imaging of Arterial Cellular Recruitment and Lipid Extravasation. Bio-protocol 7(12): e2344. DOI: 10.21769/BioProtoc.2344.
分类
细胞生物学 > 组织分析 > 组织分离
免疫学 > 免疫细胞成像 > 双光子显微镜技术
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link