- EN - English
- CN - 中文
Fluorophore Labeling, Nanodisc Reconstitution and Single-molecule Observation of a G Protein-coupled Receptor
G蛋白偶联受体的荧光标记、磷脂纳米盘重建和单分子观察
发布: 2017年06月20日第7卷第12期 DOI: 10.21769/BioProtoc.2332 浏览次数: 10236
评审: Arsalan DaudiKenji SugiyamaStéphane Roméro
Abstract
Activation of G protein-coupled receptors (GPCRs) by agonist ligands is mediated by a transition from an inactive to active receptor conformation. We describe a novel single-molecule assay that monitors activation-linked conformational transitions in individual GPCR molecules in real-time. The receptor is site-specifically labeled with a Cy3 fluorescence probe at the end of trans-membrane helix 6 and reconstituted in phospholipid nanodiscs tethered to a microscope slide. Individual receptor molecules are then monitored over time by single-molecule total internal reflection fluorescence microscopy, revealing spontaneous transitions between inactive and active-like conformations. The assay provides information on the equilibrium distribution of inactive and active receptor conformations and the rate constants for conformational exchange. The experiments can be performed in the absence of ligands, revealing the spontaneous conformational transitions responsible for basal signaling activity, or in the presence of agonist or inverse agonist ligands, revealing how the ligands alter the dynamics of the receptor to either stimulate or repress signaling activity. The resulting mechanistic information is useful for the design of improved GPCR-targeting drugs. The single-molecule assay is described in the context of the β2 adrenergic receptor, but can be extended to a variety of GPCRs.
Keywords: G-protein coupled receptors (G蛋白偶联受体)Background
GPCRs mediate cellular communications, both locally and over long distances, especially in the endocrine system. For instance, the cellular response to hormones such as adrenaline is mediated through adrenergic receptors, of which the β2 adrenergic receptor (β2AR) is a prominent member. β2AR is expressed throughout the human body and is especially important in pulmonary, cardiac and immunological systems. Pharmacologically, agonists targeting β2AR are medically proven to alleviate acute asthma attacks, since activation of β2AR relaxes smooth muscle lining in the respiratory tracts. At the molecular level, β2AR binds extracellular ligands and transmits signals across the cell membrane to intracellular effectors, such as G proteins or β arrestin. A variety of β2AR ligands are known, and these are classified as agonists or inverse agonists, depending on whether they stimulate signaling activity or reduce the activity below the basal level, respectively (Baker, 2010). Crystallographic studies have revealed the three-dimensional structures of β2AR in both inactive (Cherezov et al., 2007) and active (Rasmussen et al., 2011) conformations. However, less is known about how the receptor converts from the inactive to active conformation during signaling and how these transitions are linked to ligand binding.
Observation of single receptor molecules can reveal spontaneous conformational transitions and the influence of ligands on conformational switching, providing a unique perspective on receptor activation (Lamichhane et al., 2015). Development of a single-molecule assay requires labeling the receptor with a fluorescent reporter group at an informative position and a method for reconstituting individual receptor molecules in a membrane-like environment. A Cy3 fluorophore attached to the cytoplasmic end of trans-membrane helix 6 is a suitable reporter of conformational transitions, since the fluorescence quantum yield is sensitive to the local protein environment, a phenomenon referred to as protein-induced fluorescence enhancement (Hwang et al., 2011; Stennett et al., 2015). Moreover, phospholipid nanodiscs provide an ideal system for reconstitution and observation of single receptor molecules (Bayburt and Sligar, 2010). Individual labeled receptors in nanodiscs can be monitored over extended time periods by total internal reflection fluorescence (TIRF) microscopy, directly revealing transitions between inactive and active-like receptor conformations. The concept of the assay is illustrated in Figure 1. Statistical analysis of a collection of receptor molecules provides the rate constants for conformational exchange and the equilibrium distribution of the two conformational states, information that is difficult to obtain otherwise. These analyses can be readily performed in the absence or presence of various β2AR ligands, providing detailed mechanistic information on the linkage of receptor activation to ligand binding. Such information should be useful in the design of improved GPCR-targeting drugs with finely tuned pharmacological efficacies and reduced side effects. Here we describe the protocols to label β2AR with a Cy3 fluorophore, to reconstitute the labeled receptor in nanodiscs, to attach receptor-nanodisc complexes to a microscope slide and the procedures used to record and analyze single-molecule TIRF microscopy data. Although we describe these methods in the context of β2AR, they are equally applicable to a variety of GPCRs.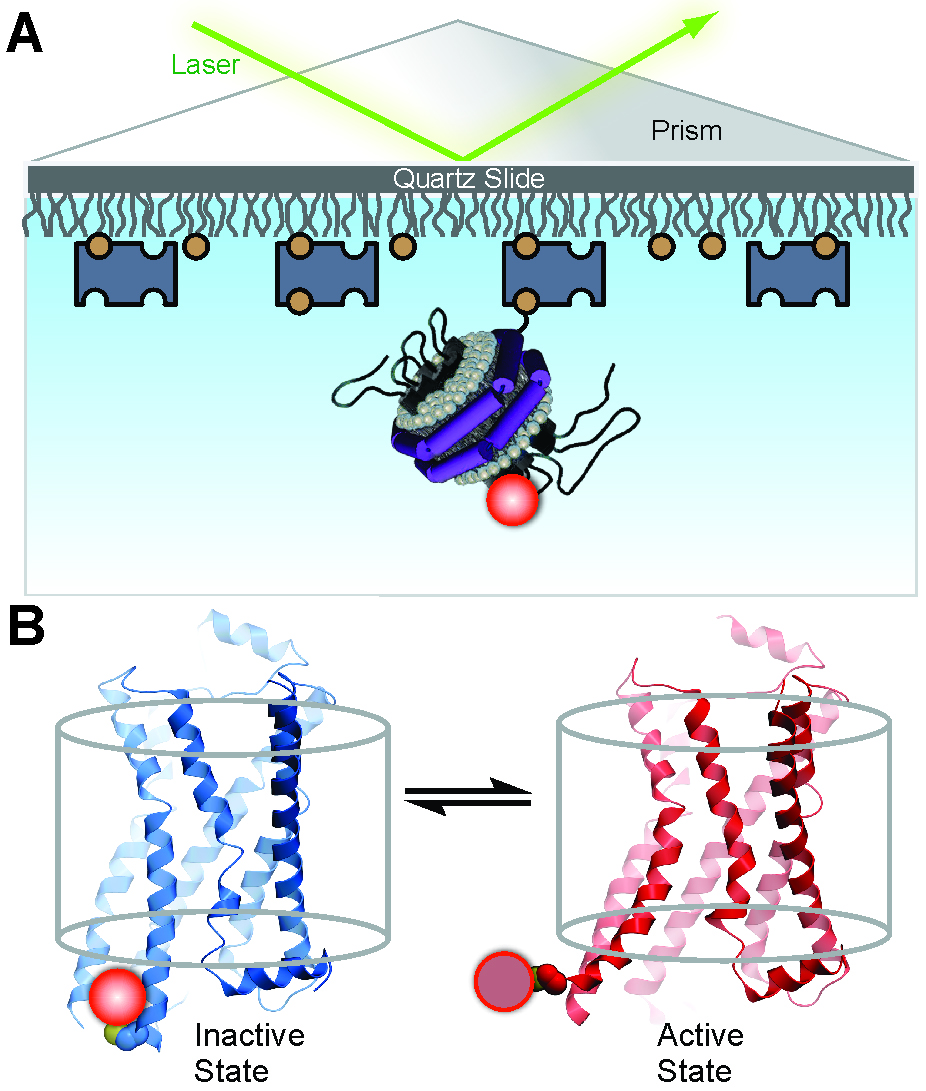
Figure 1. Experimental system to monitor conformational transitions of β2AR at the single-molecule level (reproduced from Lamichhane et al., 2015). A. An individual receptor molecule (black) labeled with Cy3 (red sphere) incorporated in a phospholipid nanodisc is tethered to a quartz surface coated with polyethylene glycol (wavy lines) via biotin (orange circles) and streptavidin (dark blue rectangles). The labeled receptor is illuminated in the evanescent field of a totally internally reflected 532 nm laser beam (green). The cartoon of the receptor-nanodisc complex is adapted from Bayburt and Sligar (2010). B. Expanded view of a single receptor-nanodisc complex, showing the receptor exchanging between inactive (blue) and active (red) conformations, with corresponding changes in the local environment of the Cy3 probe attached to Cys265 (light or dark red spheres, respectively). The transparent cylinder represents an abstraction of the lipid bilayer.
Materials and Reagents
- PD-10 desalting column (GE Healthcare, catalog number: 17085101 )
- 2 ml Eppendorf tube
- Quartz microscope slides 1” x 3” x 1 mm thick, with a small diameter hole drilled at each end (http://finkenbeiner.com/quartzslides.php)
- Microscope cover slips 22 x 40-1 (Fisher Scientific, catalog number: 12-545-C )
- Double sided tape (Scotch, 3M)
- Pipette tip
- 100 kDa MWCO vivaspin 2 concentrators polyethersulfon (PES) membrane (Sartorius, catalog number: VS1041 )
- Cy3 maleimide, mono-reactive dye (GE Healthcare, catalog number: PA13131 )
- Timolol maleate salt (Sigma-Aldrich, catalog number: T6394 )
- cOmpleteTM, Mini, EDTA-free protease inhibitor cocktail (Roche Diagnostics)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Talon metal affinity resin (Takara Bio, catalog number: 635502 )
- Imidazole (Sigma-Aldrich, catalog number: I5513 )
- DMSO (Thermo Fisher Scientific, InvitrogenTM, catalog number: D12345 )
- SDS-PAGE gels (Invitrogen NuPAGE Bis-Tris Pre-cast gels)
- Purified membrane scaffold protein 1 (MSP1), expressed in E. coli, as described (Ritchie et al., 2009)
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (Avanti Lipids Polar, catalog number: 850457P )
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS) (Avanti Lipids Polar, catalog number: 840034P )
- 16:0 biotinyl Cap PE (Avanti Lipids Polar, catalog number: 870277P )
- Bio-beads SM-2 resin (Bio-Rad Laboratories, catalog number: 1523920 )
- Ni-NTA resin (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 88222 )
- Glycerol (Sigma-Aldrich, catalog number: G6279 )
- Grease (Borer Chemie, catalog number: glisseal N )
- Neutravidin (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 31000 )
- 4-(2-hydroyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: H3375 )
- Magnesium chloride (MgCl2) (anhydrous) (Sigma-Aldrich, catalog number: M8266 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Ethylenediaminetetraacetic acid (EDTA) (0.5 M solution) (Sigma-Aldrich, catalog number: 03690 )
- N-dodecyl- β-D-maltopyranoside (DDM) (Anatrace, catalog number: D310 )
- Cholesteryl hemisuccinate (CHS) (Sigma-Aldrich, catalog number: C6512 )
- ATP (Sigma-Aldrich, catalog number: A26209 )
- Phosphate-buffered saline (PBS) (Fisher Scientific, catalog number: 70-011-044 )
- Trolox (Acros Organics, catalog number: 218940050 )
- Glucose (Sigma-Aldrich, catalog number: 158968 )
- Glucose oxidase (Sigma-Aldrich, catalog number: G2133 )
- Catalase (Sigma-Aldrich, catalog number: C3155 )
- Low salt wash buffer (see Recipe)
- High salt wash buffer (see Recipe)
- Solubilization buffer (see Recipe)
- Wash buffer 1 (see Recipe)
- Wash buffer 2 (see Recipe)
- Labeling buffer (see Recipe)
- SEC elution buffer (see Recipe)
- IMAC elution buffer (see Recipe)
- Final buffer (see Recipe)
- Imaging buffer (see Recipe)
Equipment
- 100 ml glass tissue homogenizer (Sigma-Aldrich, catalog number: T2567 )
- Beckman Ultra centrifuge
- Ti45 rotor (Beckman Coulter, model: Type 45 Ti , catalog number: 339160)
- Ti70 rotor (Beckman Coulter, model: Type 70 Ti , catalog number: 337922)
- Beckman X-12R centrifuge(Beckman Coulter, model: Allegra® X-12R ) or equivalent
- AKTAxpress FPLC system (GE Healthcare, model: AKTAxpress )
- Superdex 200 Increase 100/300 size exclusion column (GE Healthcare, catalog number: 17517501 )
- Chromatography column packed with 1-2 ml Ni-NTA agarose beads (referred to below as Ni column)
- Axiovert 200 microscope (Carl Zeiss, model: Axiovert 200 ) or equivalent
- Water-immersion C-Apochromat 63x/1.2 W objective (Carl Zeiss, model: C-Apochromat 63x/1.2 W Corr ) or equivalent
- Charge-coupled device (EMCCD) camera (Andor Technology, model: DU-897E iXon+ EMCCD ) or equivalent
- 532 nm (green) laser (CrystaLaser, catalog number: CL532-050-S ) or equivalent
Software
- Data acquisition software (available from https://cplc.illinois.edu/software/)
- MATLAB scripts
- Igor software
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Lamichhane, R., Liu, J. J., Pauszek III, R. F. and Millar, D. P. (2017). Fluorophore Labeling, Nanodisc Reconstitution and Single-molecule Observation of a G Protein-coupled Receptor. Bio-protocol 7(12): e2332. DOI: 10.21769/BioProtoc.2332.
分类
生物化学 > 蛋白质 > 单分子活性 > 成像
生物化学 > 蛋白质 > 标记
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













