- EN - English
- CN - 中文
Explant Methodology for Analyzing Neuroblast Migration
采用外植体方法分析神经母细胞迁移
发布: 2017年05月05日第7卷第9期 DOI: 10.21769/BioProtoc.2249 浏览次数: 10040
评审: Khyati Hitesh ShahAnonymous reviewer(s)
Abstract
The subventricular zone (SVZ) in the mammalian forebrain contains stem/progenitor cells that migrate through the rostral migratory stream (RMS) to the olfactory bulb throughout adulthood. SVZ-derived explant cultures provide a convenient method to assess factors regulating the intermediary stage of neural stem/progenitor cell migration. Here, we describe the isolation of SVZ-derived RMS explants from the neonatal mouse brain, and the conditions required to culture and evaluate their migration.
Keywords: Neuroblasts (神经母细胞)Background
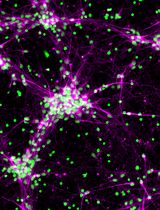
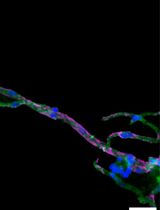
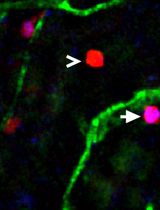
The adult mammalian forebrain contains a neurogenic niche that lies alongside the lateral ventricle in rodents and humans alike, and is aptly named the subventricular zone (SVZ). In rodents, the SVZ is a thin ‘wedge’ of cells, covering the entire wall of the lateral ventricle (Mirzadeh et al., 2010; Paez-Gonzalez et al., 2014; Dixon et al., 2016). Within the SVZ the slow-dividing astrocyte-like type B cells differentiate into rapidly dividing type C neural progenitor cells (also known as transit amplifying cells) that give rise to doublecortin-positive type A neuroblasts, although oligodendrocytes and astrocytes are also capable of being produced (Garcia-Verdugo et al., 1998; Tavazoie et al., 2008; Rikani et al., 2013). In rodents, there are an estimated 10,000 to 30,000 neuroblasts produced daily. These neuroblasts form chains as they migrate through the rostral migratory stream (RMS) to the olfactory bulb (Lois and Alvarez-Buylla, 1994; Sun et al., 2010). Ablation studies suggest it takes approximately 2 days for fast dividing type C neural progenitor cells to populate the SVZ, and an additional 2.5 days for neuroblasts to appear (Doetsch et al., 1999). A small percentage of these neuroblasts are capable of migrating ectopically out of the RMS into surrounding tissues in naïve mice; however, this phenomenon is drastically increased following brain injury (Dixon et al., 2016). The ability of neuroblasts to redirect their migratory routes towards damaged tissues has been shown to have beneficial effects on brain recovery (Li et al., 2010; Dixon et al., 2015), which can occur as early as 3 days post-injury (Ramaswamy et al., 2005; Dixon et al., 2016).
The self-renewal capacity of stem cells in culture was first identified in 1992 by Reynolds and Weiss (Reynolds and Weiss, 1992). The authors used fine dissection to harvest a small piece of the adult mouse striatum, before trypsinizing, dissociating and culturing. This original protocol, and subsequent variations, are now widely used to grow neurospheres or monolayer cultures to assess factors regulating stem cell survival, proliferation and/or differentiation into neurons (Theus et al., 2012). These culturing systems rely on the presence of growth factors (i.e., fibroblast and epidermal growth factors) to maintain proliferative states, whereas the withdrawal of these factors induces rapid differentiation into mature neurons. Unfortunately, these conditions limit the ability to analyze factors that regulate type A neuroblasts, a transient stage between the stem cell and neuron. To counteract this limitation; pieces of SVZ-derived tissue can be harvested and cultured as explants in a Matrigel containing laminin and collagen, which maintains the neural stem cells in their neuroblast state, allowing them to migrate (Ward and Rao, 2005; Dixon et al., 2016). Furthermore, neuroblast migration from cultured SVZ explants has similar characteristics to those observed in the RMS. Here, we describe an RMS explant methodology, modified from Ward and colleague (Leong et al., 2011), used to study chain migration of SVZ-derived neuroblasts.
Materials and Reagents
- 35 mm cell culture dishes with 4 internal wells, each with a diameter of 10 mm (Greiner Bio One International, catalog number: 627170 )
- 10 cm cell culture dishes (Corning, catalog number: 353003 )
- Pre-chilled (-20 °C) tissue culture pipette filter tips
- 10 µl tips (Corning, Axygen®, catalog number: TF-300-R-S )
- 200 µl tips (Corning, Axygen®, catalog number: TF-200-R-S )
- 1,000 µl tips (Corning, Axygen®, catalog number: TF-1000-R-S )
- 5 ml serological pipettes and pipette-boy (VWR, catalog number: 612-3702 )
- 1.5 ml Eppendorf microcentrifuge tubes (VWR, catalog number: 211-0007 )
- 3.2 ml disposable transfer pipette (Thermo Fisher Scientific, catalog number: BER202-1S )
- 1 ml insulin syringe with detachable needle (BD, catalog number: 329651 )
- Ice tray and ice as available
- Small biohazard bags as available
- Marker pen (e.g., Sharpie) for labelling cell culture plates
- 1-2 postnatal day old C57Bl/6 wildtype mice (Animal Resources Centre, Australia) or from local animal supplier
- Absolute ethanol (Sigma-Aldrich, catalog number: 24102 )
- Hanks balanced salt solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14170112 )
- Growth factor reduced Matrigel (Corning, catalog number: 356230 )
- Neurobasal medium (Thermo Fisher Scientific, GibcoTM, catalog number: 21103049 )
- B27 supplement x50 (Thermo Fisher Scientific, GibcoTM, catalog number: 17504044 )
- 200 mM glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030081 )
- Penicillin/streptomycin (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Recombinant murine fibroblast growth factor (FGF) basic 1 mg/ml (PeproTech, catalog number: 450-33 )
- Recombinant murine epidermal growth factor (EGF) 1 mg/ml (PeproTech, catalog number: 315-09 )
- Paraformaldehyde (Sigma-Aldrich, catalog number: 158127 )
- Sodium phosphate dibasic (Na2HPO4) (Chem Supply, catalog number: SA026 )
- Sodium dihydrogen phosphate monohydrate (NaH2PO4·H2O) (Chem Supply, catalog number: SO03310500 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: S5881 )
- DAPI (Thermo Fisher Scientific, GibcoTM, catalog number: D1306 )
- Donkey serum (Sigma Aldrich, catalog number: D9663 )
- Triton X-100 (Sigma-Aldrich, catalog number: X100 )
- Goat anti-doublecortin (DCX) antibody (Santa Cruz Biotechnology, catalog number: sc-8066 or sc-271390 )
Note: The authors used sc-8066 , this antibody has been discontinued. A suggested alternative from Santa Cruz Biotechnology is sc-271390 . - Cy3-conjugated donkey anti-goat antibody (Jackson ImmunoResearch, catalog number: 705-165-147 )
- 80% ethanol solution (see Recipes)
- Complete neurobasal medium (see Recipes)
- Complete Matrigel (see Recipes)
- 0.1 M phosphate buffered saline (PBS) (see Recipes)
- 4% paraformaldehyde (PFA) (see Recipes)
- PBS containing DAPI (see Recipes)
- Blocking buffer (see Recipes)
- Primary antibody (see Recipes)
- Secondary antibody (see Recipes)
Equipment
- Pipettes
20 µl pipette (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4642050 )
200 µl pipette (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4642080 )
1,000 µl pipette (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4642090 ) - Biosafety cabinet, any brand/model
- Bottles, sterile glass or plastic, any brand, for tissue culture medium storage
- Millipore StericupTM sterile vacuum filter units (EMD Millipore, catalog number: SCGPU01RE )
- Dissection microscope (Olympus, or similar) with lamp (or cold light source)
- Dissecting scissors, 12.5 cm long, straight (Coherent Scientific, catalog number: 15922 )
- Iris scissors 10 cm long, 30° angle, supercut (Coherent Scientific, catalog number: 500046 )
- Dressing forceps, 15.5 cm long (Coherent Scientific, catalog number: 500363 )
- 2 x Dumont forceps #3, 12 cm long, 0.08 x 0.04 mm tips (Coherent Scientific, catalog number: 500337 )
- Dissecting spatula, 140 mm long, 3 wide mm blade (World Precision Instruments, catalog number: 501772 )
- Scalpel handle No. 3 with scalpel blade No. 15 (Coherent Scientific , catalog numbers: 500236 and 500242 )
- Dumont forceps #5, 11 cm long, 0.06 x 0.01 mm tips (Coherent Scientific, catalog number: 14095 )
- Humidified tissue culture incubator (5% CO2, 37 °C), any brand/model
- Refrigerator (4 °C), any brand/model
- Freezer (-20 °C), any brand/model
- Inverted fluorescent microscope and digital camera (Olympus, model: IX81 or similar)
Software
- Axiovision software v4.1 (Zeiss, Thornwood, NY) or similar
- GraphPad Prism (v4.03)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Dixon, K. J., Turbic, A., Turnley, A. M. and Liebl, D. J. (2017). Explant Methodology for Analyzing Neuroblast Migration. Bio-protocol 7(9): e2249. DOI: 10.21769/BioProtoc.2249.
分类
干细胞 > 成体干细胞 > 神经干细胞
细胞生物学 > 细胞运动 > 细胞迁移
细胞生物学 > 组织分析 > 组织分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link