- EN - English
- CN - 中文
Isolation, Culturing, and Differentiation of Primary Myoblasts from Skeletal Muscle of Adult Mice
成年小鼠骨骼肌的原代成肌细胞分离、培养和分化
发布: 2017年05月05日第7卷第9期 DOI: 10.21769/BioProtoc.2248 浏览次数: 28441
评审: Antoine de MorreeXiaoyi ZhengRakesh Bam
Abstract
Myogenesis is a multi-step process that leads to the formation of skeletal muscle during embryonic development and repair of injured myofibers. In this process, myoblasts are the main effector cell type which fuse with each other or to injured myofibers leading to the formation of new myofibers or regeneration of skeletal muscle in adults. Many steps of myogenesis can be recapitulated through in vitro differentiation of myoblasts into myotubes. Most laboratories use immortalized myogenic cells lines that also differentiate into myotubes. Although these cell lines have been found quite useful to delineating the regulatory mechanisms of myogenesis, they often show a great degree of variability depending on the origin of the cells and culture conditions. Primary myoblasts have been suggested as the most physiologically relevant model for studying myogenesis in vitro. However, due to their low abundance in adult skeletal muscle, isolation of primary myoblasts is technically challenging. In this article, we describe an improved protocol for the isolation of primary myoblasts from adult skeletal muscle of mice. We also describe methods for their culturing and differentiation into myotubes.
Keywords: Myoblast (成肌细胞)Background
Myogenesis is a complex and highly orchestrated process that involves the determination of multipotential mesodermal cells to give rise to myoblasts, exit of myoblasts from the cell cycle, and their eventual differentiation into skeletal muscle fibers. Myogenesis is regulated by the sequential expression of myogenic regulatory factors (MRFs), a group of basic helix-loop-helix transcription factors that include Myf-5, MyoD, myogenin, and MRF4. Myf-5 and MyoD are the primary MRFs required for the formation, proliferation, and survival of myoblasts, whereas other MRFs such as myogenin and MRF-4 act late during myogenesis, activating gene expression of contractile proteins and other structural and metabolic proteins (Buckingham et al., 2003; Bentzinger et al., 2012).
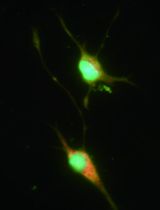
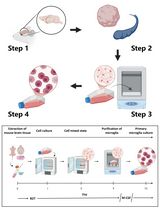
Myogenesis is also regulated by a number of transcription factors and several noncoding RNAs, which act at specific steps including commitment of progenitor (satellite) cells to myogenic lineage and myoblast proliferation, differentiation, and fusion (Yin et al., 2013; Simionescu-Bankston and Kumar, 2016). Initial experiments for studying the role of various regulatory proteins in myogenesis are performed using cultured myoblasts. There are several myoblastic cell lines (e.g., C2C12, L6, BC3H1, and MM14) that differentiate into myotubes upon incubation in differentiation medium. These cell lines have also been used to establish myotube cultures to investigate the effects of various molecules on myotube growth and atrophy. However, there is often some degree of variability in results potentially due to the origin of cells, culture conditions, and passage number. The use of primary myoblasts is highly recommended because they are devoid of the side-effects characteristic of the immortalization process and their physiological relevance to the living organisms. Primary myoblasts can be isolated from the skeletal muscle of neonatal or adult mice. However, the process of isolation of myoblasts from neonatal muscle is more complex because it also requires Percoll density gradient centrifugation (Dogra et al., 2006). Some investigators also use fluorescence-activated cell sorting (FACS) approach to isolate primary myoblasts from digested muscle tissues especially to study regulation of quiescence and activation of these cells. However, FACS sorting is an expensive approach which requires several negative and positive selection antibodies and a cell sorter machine. Moreover, the yield of myoblasts is generally low and there are always chances of contamination during isolation of purified myoblasts by FACS technique. In our laboratory, we have adapted and standardized a previously published protocol (Rando and Blau, 1994) for the isolation of myoblasts from skeletal muscle of adult mice. This protocol is highly efficient for the generation of a large amount of purified myoblasts from skeletal muscle of adult mice (Ogura et al., 2015; Hindi and Kumar, 2016). The purity of the myoblasts can be assayed by immunostaining of the cells for Pax7 and MyoD proteins which are expressed in undifferentiated myoblasts. Moreover, primary myoblasts isolated using this protocol efficiently differentiate into multinucleated myotubes on incubation in differentiation medium and myotubes can be readily visualized by phase contrast microscopy or after immunostaining for myosin heavy chain (MyHC), a protein expressed in differentiated muscle cells (Hindi et al., 2014; Bohnert et al., 2016). Finally, like myogenic cell lines, the purified primary myoblasts can be stored in liquid nitrogen or -80 °C for unlimited time and can be regrown whenever required.
Materials and Reagents
- Sterilization pouches (Fisher Scientific, catalog number: 01-812-51 )
- 100 x 20 mm-Petri dishes (Corning, catalog number: 430167 )
- 6-well plates (Corning, Falcon®, catalog number: 353046 )
- 1.5 ml Eppendorf tubes (USA Scientific, catalog number: 1615-5510 )
- 0.22 μm filter (EMD Millipore, catalog number: SLGP033RS )
- 15 ml sterile tubes (VWR, catalog number: 89004-368 )
- 1 ml pipette tip
- Parafilm
- 10 ml serological pipette (Santa Cruz Biotechnology, catalog number: sc-200281 )
- 70 µm strainer (Fisher Scientific, catalog number: 22-363-548 )
- 50 ml sterile tubes (VWR, catalog number: 89004-364 )
- 30 µm filters (Milteny Biotech, catalog number: 130-041-407 )
- 0.45 μm filter (EMD Millipore, catalog number: SLHV033RS )
- 24-well plates (Corning, Falcon®, catalog number: 353047 )
- Slip-tip syringe (BD, catalog number: 302833 )
- Sterile cell scraper (Corning, Falcon®, catalog number: 353085 )
- Adult mice (Mus musculus; 6-8-weeks old) (see Notes 7 and 8)
- 2,2,2-tribromoethanol (Avertin) (Sigma-Aldrich, catalog number: T48402 )
- 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056 )
- Phosphate buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2153 )
- Primary antibody anti-Pax7 (mouse) (Developmental Studies Hybridoma Bank, catalog number: Pax7 )
- Primary antibody anti-MyoD (rabbit) (Santa Cruz Biotechnology, catalog number: sc-304 )
- Primary antibody anti-MyHC (mouse) (Developmental Studies Hybridoma Bank, catalog number: MF-20 )
- Secondary antibody goat anti-rabbit Alexa Fluor® 488 conjugate (Thermo Fisher Scientific, Invitrogen, catalog number: A-11034 )
- Secondary antibody goat anti-mouse Alexa Fluor® 568 conjugate (Thermo Fisher Scientific, Invitrogen, catalog number: A-11004 )
- 100% ethanol (Decon Labs, catalog number: 2701 )
- Matrigel (Corning, catalog number: 354234 )
- Dulbecco’s modified Eagle’s medium (DMEM) high glucose, pyruvate (Thermo Fisher Scientific, GibcoTM, catalog number: 11995065 )
- Collagenase II (Worthington Biochemical, catalog number: LS004176 )
- Ultra-pureTM water (Thermo Fisher Scientific, InvitrogenTM, catalog number: 10977015 )
- Penicillin-streptomycin (Pen/Strep) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES) (1 M) (Thermo Fisher Scientific, GibcoTM, catalog number: 15630080 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10437028 )
- Recombinant human fibroblast growth factor-basic (bFGF) (PeproTech, catalog number: 100-18B )
- Tris base (Fisher Scientific, catalog number: BP152-5 )
- F-10 Nutrient mixture (Thermo Fisher Scientific, GibcoTM, catalog number: 11550043 )
- Dulbecco’s modified Eagle’s medium (DMEM) (ATCC, catalog number: 30-2002 )
- Horse serum (Thermo Fisher Scientific, GibcoTM, catalog number: 26050088 )
- Dimethyl sulfoxide (DMSO) (Fisher Scientific, catalog number: BP231-100 )
- Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: P6148 )
- 100% Triton X-100 (Fisher Scientific, catalog number: BP151-500 )
- 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich, catalog number: D8417 )
- 70% ethanol (see Recipes)
- 10% Matrigel (see Recipes)
- Collagenase II (see Recipes)
- Digestion medium (see Recipes)
- Collection/washing/mincing solution (see Recipes)
- Neutralization/isolation media (see Recipes)
- Basic fibroblast growth factor (bFGF) (see Recipes)
- Myoblast growth medium (MGM) (see Recipes)
- Post isolation washing medium (see Recipes)
- Differentiation medium (DM) (see Recipes)
- Freezing medium (see Recipes)
- 4% paraformaldehyde (PFA) (see Recipes)
- 0.3% Triton X-100 (see Recipes)
- 10% Triton X-100 (see Recipes)
- Blocking solution (see Recipes)
- DAPI (see Recipes)
Equipment
- Autoclave
- Dissection tools: Sterilized/autoclaved scissors and forceps
- Biosafety cabinet (Thermo Fisher Scientific, Thermo ScientificTM, model: 1300 Series Class II , Type A2)
- Bench top centrifuge for 1.5 ml Eppendorf tubes (Eppendorf, model: 5424/5424 R )
- Bench top centrifuge for 15 ml and 50 ml tubes (Eppendorf, model: 5702/5702 R/5702 RH )
- Heated incubator shaker (Eppendorf, New BrunswickTM, model: Excella E24 )
- CO2 incubator (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 3578 )
- Microscope (Nikon Instruments, model: Eclipse TE2000 )
- Water bath (Thermo Fisher Scientific, model: Model 215 , catalog number: 15-462-15Q)
- 500 ml bottle
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Hindi, L., McMillan, J. D., Afroze, D., Hindi, S. M. and Kumar, A. (2017). Isolation, Culturing, and Differentiation of Primary Myoblasts from Skeletal Muscle of Adult Mice. Bio-protocol 7(9): e2248. DOI: 10.21769/BioProtoc.2248.
分类
神经科学 > 细胞机理 > 细胞分离和培养
干细胞 > 成体干细胞 > 肌肉干细胞
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link