- EN - English
- CN - 中文
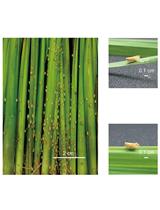
Bioassay of Xanthomonas albilineans Attachment on Sugarcane Leaves
采用生物测定法研究甘蔗叶白纹黄单胞菌的附着
发布: 2017年01月20日第7卷第2期 DOI: 10.21769/BioProtoc.2111 浏览次数: 10272
评审: Zhaohui LiuShahin S. AliAnonymous reviewer(s)
Abstract
Sugarcane (interspecific hybrids of Saccharum species) is an economically important crop that provides 70% of raw table sugar production worldwide and contributes, in some countries, to bioethanol and electricity production. Leaf scald, caused by the bacterial plant pathogen Xanthomonas albilineans, is one of the major diseases of sugarcane. Dissemination of X. albilineans is mainly ensured by contaminated harvesting tools and infected stalk cuttings. However, some strains of this pathogen are transmitted by aerial means and are able to survive as epiphytes on the sugarcane phyllosphere before entering the leaves and causing disease. Here we present a protocol to estimate the capacity of attachment of X. albilineans to sugarcane leaves. Tissue-cultured sugarcane plantlets were immersed in a bacterial suspension of X. albilineans and leaf attachment of X. albilineans was determined by two methods: leaf imprinting (semi-quantitative method) and leaf washing/homogenization (quantitative method). These methods are important tools for evaluating pathogenicity of strains/mutants of the sugarcane leaf scald pathogen.
Keywords: Attachment (附着)Background
The mechanisms that govern the interactions between X. albilineans and its host plant (the sugarcane) are not well known. Albicidin, a phytotoxin produced by albilineans, is the only molecular factor which has been demonstrated to play a role in pathogenicity of this pathogen (Birch, 2001). However, pathogenicity of X. albilineans doesn’t completely depend on albicidin. Albicidin-deficient mutants are still able to colonize efficiently the sugarcane stalk and to produce disease symptoms (Birch, 2001; Rott et al., 2011). Studies using full grown sugarcane are space and time consuming. Bioassays using miniaturized plants (tissue-cultured plants) or detached leaf bioassays can be very useful because they are less space consuming and they allow the study of plant-pathogen interactions in controlled environments. In vitro propagation of plants is widely used to rapidly propagate disease-free planting material under controlled conditions (Kumar and Reddy, 2011). Additionally, leaf imprinting has been widely used to study the ecology of bacteria associated with the phyllosphere (Hirano and Upper, 2000; Yadav et al., 2010). However, to our knowledge, these techniques have never been associated to decipher pathogenicity of bacterial plant pathogens. To identify additional pathogenicity factors of X. albilineans, especially factors involved in the early phases of infection (epiphytic phase), we developed a new miniaturized bioassay using tissue cultured sugarcane plantlets. Attachment of X. albilineans to sugarcane leaves under axenic condition was reproduced (Fleites et al., 2013; Mensi et al., 2016). This bioassay will permit the rapid testing of leaf attachment capacity of wild type and mutant strains of the pathogen causing leaf scald disease, but also of other bacteria colonizing the sugarcane leaf canopy.
Materials and Reagents
- Sterile scalpels blades (Swan Morton, catalog numbers: n° 11 and n° 24 )
- Sterilized pipette tips
200 µl (Thermo Fischer Scientific, Fischer Scientific, catalog number: 02-681-2 )
1,000 µl (Thermo Fischer Scientific, Fischer Scientific, catalog number: 02-681-4 ) - Sterile plastic loops (Greiner Bio One, catalog number: 731171 )
- Falcon 15 ml conical centrifuge tubes (SARSTEDT, catalog number: 62.554.502 )
- Soft tissue (Orapi Hygiène, catalog number: 186 )
- Disposable, sterile splinter removers/tweezers – 11.1 cm. (4 ¼ in.) (TSIC Solution, catalog number: UTIL-1037 )
- 90 x 15 mm Petri dishes (Corning, GosselinTM, catalog number: BP93B-15 )
- 1.5 ml microcentrifuge tube (SARSTEDT, catalog number: 72.690.001 )
- Disposable pellet pestle for 1.5 ml centrifuge tube (Kimble Chase Life Science and Research Products, catalog number: 749521-1500 )
- Sugarcane plantlets (cultivar CP68-1026 susceptible to leaf scald disease of sugarcane)
- Xanthomonas albilineans wild type strains and mutants affected in pathogenicity (grown for 4 to 5 days on Wilbrink medium + appropriate antibiotics); for characteristics of mutants, see Fleites et al. (2013) and Mensi et al. (2016)
- Sterile distilled water
- Tween 20 (Sigma-Aldrich, catalog number: P2287 )
- Sucrose (Merck Millipore, catalog number: 107687 )
- Peptone (BD, BactoTM, catalog number: 211677 )
- Potassium phosphate, dibasic, trihydrate (K2HPO4·3H2O) (EMD Millipore, Calbiochem®, catalog number: 529567 )
- Magnesium sulfate heptahydrate (MgSO4·7H2O) (EMD Millipore, catalog number: 105886 )
- Sodium sulfite (Na2SO3) (EMD Millipore, catalog number: 106657 )
- Agar (BD, BactoTM, catalog number: 214010 )
- Potassium bromide, KBr (Sigma-Aldrich, catalog number: P0838 )
- Benomyl (Sigma-Aldrich, catalog number: 381586 )
- Cycloheximide (Sigma-Aldrich, catalog number: C1988 )
- Ethanol (Sigma-Aldrich, catalog number: 32294 )
Note: This product has been discontinued. - Cephalexin (Sigma-Aldrich, catalog number: C0675000 )
- Novobiocin (Sigma-Aldrich, catalog number: 1475008 )
- Kasugamycin (Sigma-Aldrich, catalog number: 19408-46-9 )
- Ammonium nitrate (NH4NO3) (Sigma-Aldrich, catalog number: A3795 )
- Potassium nitrate (KNO3) (Sigma-Aldrich, catalog number: P8291 )
- Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) (Sigma-Aldrich, catalog number: C2786 )
- Magnesium sulfate heptahydrate (MgSO4·7H2O) (Sigma-Aldrich, catalog number: 63138 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P5405 )
- Boric acid (H3BO3) (Sigma-Aldrich, catalog number: B6768 )
- Manganese(II) sulfate monohydrate (MnSO4·H2O) (Sigma-Aldrich, catalog number: M7899 )
- Zinc sulfate heptahydrate (ZnSO4·7H2O) (Sigma-Aldrich, catalog number: Z1001 )
- Potassium iodide (KI) (Sigma-Aldrich, catalog number: P8166 )
- Ammonium molybdate tetrahyddrate ((NH4)6Mo7O24·4H2O) (Sigma-Aldrich, catalog number: M1019 )
- Copper(II) nitrate trihydrate (Cu(NO3)2·3H2O) (Sigma-Aldrich, catalog number: 61194 )
- Iron(II) sulfate heptahydrate (FeSO4·7H2O) (EMD Millipore, catalog number: 103965 )
- Na2EDTA·2H2O (Sigma-Aldrich, catalog number: E5134 )
- Nicotinic acid (Sigma-Aldrich, catalog number: N4126 )
- Pyridoxol hydrochloride (Sigma-Aldrich, catalog number: P6280 )
- Myo-inositol (Sigma-Aldrich, catalog number: I7508 )
- Thiamine dichloride (Sigma-Aldrich, catalog number: T1270 )
- Phytagel (Sigma-Aldrich, catalog number: P8169 )
- Wilbrink medium (WM) (see Recipes)
- Wilbrink Selective Davis (WSD) medium (see Recipes)
- Macronutrients (see Recipes)
- Micronutrients (see Recipes)
- Ferric EDTA (see Recipes)
- Fuji vitamins (see Recipes)
- Nutritive medium for growth of sugarcane plantlets (see Recipes)
Equipment
- Growth chamber
- 200 x 20 mm Pyrex test tubes with cap (Legallais, catalog number: 761224 )
- 200 mm long tweezers with blunt tips (VWR, catalog number: 82027-436 )
- Micropipettes
20-200 µl (Eppendorf, catalog number: 3120000054 )
100-1,000 µl (Eppendorf, catalog number: 3120000062 ) - Scalpels (Swan Morton, catalog numbers: N° 3G S/S and N° 4G S/S )
- Incubator for microbiology (Memmert, model: B40 )
Note: This product has been discontinued. - Benchtop vortex (Scientific Industries, model: Vortex Genie 2 )
- Spectrophotometer (Eppendorf Biophotometer)
- 250 or 500 ml wide neck Erlenmeyer flasks (Borosilicate glass) (Duran, catalog number: 21 226 36 or 21 226 44 )
- Laminar flow cabinet or sterile hood
- Autoclave
Software
- Package R, version 2.14.1 (R Development Core Team)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Mensi, I., Daugrois, J. and Rott, P. (2017). Bioassay of Xanthomonas albilineans Attachment on Sugarcane Leaves. Bio-protocol 7(2): e2111. DOI: 10.21769/BioProtoc.2111.
分类
植物科学 > 植物免疫 > 病害生物测定
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link