- EN - English
- CN - 中文
Aggregation Prevention Assay for Chaperone Activity of Proteins Using Spectroflurometry
使用荧光光谱法测定蛋白质聚集抑制实验中分子伴侣的活性
(*contributed equally to this work, §Deceased) 发布: 2017年01月20日第7卷第2期 DOI: 10.21769/BioProtoc.2107 浏览次数: 12677
评审: Valentine V TrotterSoazig Le Guyon Anonymous reviewer(s)

相关实验方案
从大肠杆菌中大规模纯化III型毒素-抗毒素核糖蛋白复合物及其成分,用于生物物理学研究
Parthasarathy Manikandan [...] Mahavir Singh
2023年07月05日 2169 阅读
基于蛋白筛选策略从合成文库中分离抗原特异性纳米抗体:结合 MACS 的酵母展示筛选与 FLI-TRAP 方法
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
2026年01月20日 420 阅读
Abstract
The ability to stabilize other proteins against thermal aggregation is one of the major characteristics of chaperone proteins. Molecular chaperones bind to nonnative conformations of proteins. Folding of the substrate is triggered by a dynamic association and dissociation cycles which keep the substrate protein on track of the folding pathway (Figure 1). Usually molecular chaperones exhibit differential affinities with different conformations of the substrate. With the exception of the sHsp family of molecular chaperones, the shift from a high-affinity binding state to the low-affinity release state is triggered by ATP binding and hydrolysis (Haselback and Buchner, 2015). Aggregation prevention assay is a simple, yet definitive assay to determine the chaperone activity of heat labile proteins such as Maltodextrin glucosidase (MalZ), Citrate Synthase (CS) and NdeI. This is based on the premise that proteins with chaperone like activity should prevent protein substrates (MalZ, CS and NdeI) from thermal aggregation. Here, we describe a detailed protocol for aggregation prevention assay using two different chaperone proteins, resistin and MoxR1, identified from our lab. Resistin, a human protein (hRes) and MoxR1 a Mycobacterium tuberculosis protein were analysed for their effect on prevention of MalZ/Citrate Synthase (CS)/NdeI aggregation.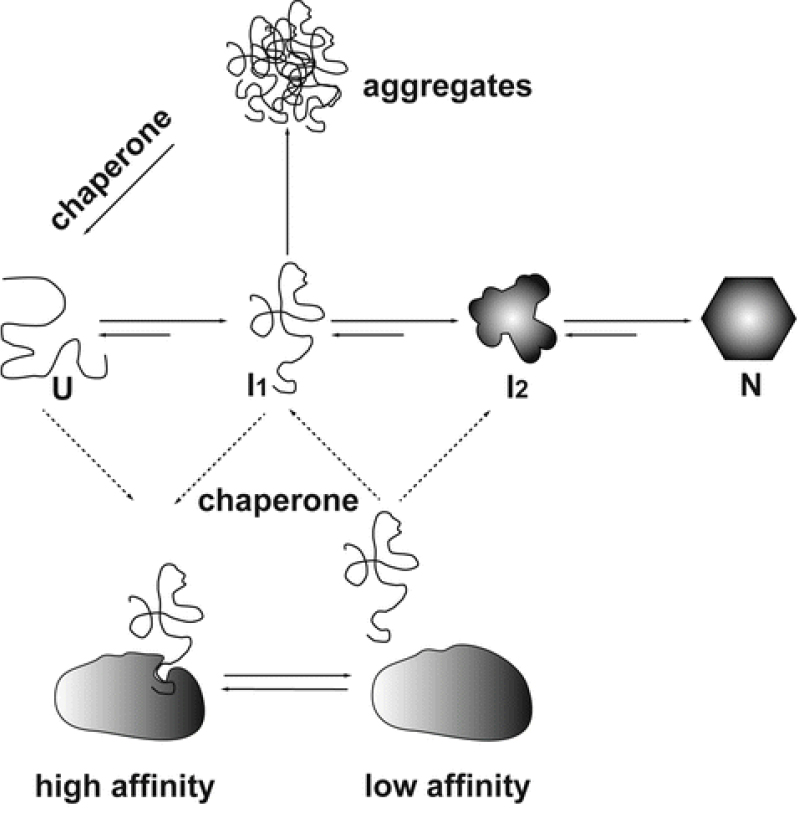
Figure 1. Mechanism of action of molecular chaperones. Citrate synthase folds via increasingly structured intermediates (I1, I2) from the unfolded state (U) to the folded state (N). Under heat shock conditions, this process is reversed.
Background
To elucidate the chaperone activity of a protein in vitro, several methods have been developed. Primarily these methods are based on examining the enzyme function and the ability of the chaperone to refold and protect enzyme activity under heat or other stress conditions. Other method to identify and study chaperones includes in silico analysis, or co-purification with other proteins. The limitations with such methods are less reproducibility or inherent high chances of false positive results. In the current method the use of light scattering to detect prevention of protein aggregation relies on thermal stabilization of protein only from denatured state, and in the presence of a chaperone. In contrast, if aggregate is formed from native or intermediate state of the protein the amount of aggregation might increase thereby decreasing the chance of false positive. Furthermore, this method uses the purified recombinant protein for the assay and therefore, can also be used to study other chaperone proteins from other bacterial sources.
Materials and Reagents
- Pipette tips
- Microcentrifuge tube
- Parafilm
- E. coli BL21 (DE3) cells
- Citrate synthase ammonium sulfate suspension from porcine heart (Sigma-Aldrich, catalog number: C3260 )
- Ammonium sulphate
- Tris (pH 8.0) (AMRESCO, catalog number: 0497 )
- Luria Bertani agar plates
- Ampicillin (Sigma- Aldrich, catalog number: A9518 )
- Isopropyl β-D-1-thiogalactopyranoside, IPTG (Sigma-Aldrich, catalog number: I6758 )
- DNAse I (Sigma-Aldrich, catalog number: AMPD1 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2670 )
- Phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: 7626 )
- Bradford reagent (Bio-Rad Laboratories, catalog number: 5000006 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906 )
- Recombinant protein MoxR1 and hRes (1 mg/ml) (Purified in the laboratory)
- Purified recombinant protein GroEL (0.5 mg/ml) (Purified in the laboratory)
- Lysozyme (1 mg/ml) (Sigma-Aldrich, catalog number: 4919 )
- Milli-Q water
- NdeI (20,000 U/ml) (New England BioLab, catalog number: R0111 )
- Maltodextrin glucosidase protein (1 mg/ml) (Purified in the laboratory)
- EDTA (Sigma-Aldrich, catalog number: E9884 )
- Sodium phosphate (dibasic) heptahydrate (Na2HPO4·7H2O) (AMRESCO, catalog number: 0348 )
- Sodium phosphate (monobasic) anhydrous (NaH2PO4) (AMRESCO, catalog number: 0571 )
- Sodium chloride (NaCl) (AMRESCO, catalog number: 0241 )
- Imidazole (AMRESCO, catalog number: 0527 )
- Glycerol (AMRESCO, catalog number: 0854 )
- TE buffer (see Recipes)
- 20 mM sodium phosphate buffer pH 7.4 (see Recipes)
- Binding buffer (see Recipes)
- Washing buffer (see Recipes)
- Elution buffer (see Recipes)
- Dialysis buffer (see Recipes)
Equipment
- Pipette (Gilson, model: Pipetman Neo®)
- Centrifuge (Hermle Labor Technik, model: Z 326 K )
- PerkinElmer spectrofluorometer (PerkinElmer, model: LS55 ) with controlled temperature peltier block
- Incubator (Eppendorf, BrunswickTM, model: 44/44R )
- Quartz cuvette (PerkinElmer, Suprasil®, catalog number: B0631071 )
- pH meter (Thermo Fisher Scientific, Thermo Scientific, model: CyberScan Ph 510 )
- Fluorescence spectrophotometer (Agilent Technologies, model: Cary Eclipse )
Software
- Microsoft Excel
- Graphpad Prism 5
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Bhuwan, M., Suragani, M., Ehtesham, N. Z. and Hasnain, S. E. (2017). Aggregation Prevention Assay for Chaperone Activity of Proteins Using Spectroflurometry. Bio-protocol 7(2): e2107. DOI: 10.21769/BioProtoc.2107.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 结构
微生物学 > 微生物生物化学 > 蛋白质 > 相互作用
生物化学 > 蛋白质 > 相互作用 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link