- EN - English
- CN - 中文
Assessment of Cellular Redox State Using NAD(P)H Fluorescence Intensity and Lifetime
利用NAD(P)H荧光强度和寿命评估细胞氧化还原状态
发布: 2017年01月20日第7卷第2期 DOI: 10.21769/BioProtoc.2105 浏览次数: 14858
评审: Nicoletta CordaniRakesh BamAnonymous reviewer(s)
Abstract
NADH and NADPH are redox cofactors, primarily involved in catabolic and anabolic metabolic processes respectively. In addition, NADPH plays an important role in cellular antioxidant defence. In live cells and tissues, the intensity of their spectrally-identical autofluorescence, termed NAD(P)H, can be used to probe the mitochondrial redox state, while their distinct enzyme-binding characteristics can be used to separate their relative contributions to the total NAD(P)H intensity using fluorescence lifetime imaging microscopy (FLIM). These protocols allow differences in metabolism to be detected between cell types and altered physiological and pathological states.
Keywords: NADH (NADH)Background
The reduced form of the redox cofactor nicotinamide adenine dinucleotide (NADH) and its phosphorylated counterpart NADPH are intrinsically fluorescent, both absorbing light at wavelengths of 340 (± 30) nm and emitting at 460 (± 50) nm (Patterson et al., 2000). These spectral characteristics are lost upon oxidation to NAD+ or NADP+ (De Ruyck et al., 2007). The redox balances of the separate NAD and NADP pools dictate contrasting metabolic processes (Ying, 2008), as shown in Figure 1. NAD acts as an electron acceptor for the oxidation of sugar, lipid and amino acid substrates in the mitochondria by the tricarboxylic acid (TCA) cycle and as an electron donor to the electron transport chain (ETC) on the inner mitochondrial membrane (IMM), fuelling the pumping of protons into the intermembrane space to act as a power source for the synthesis of adenosine triphosphate (ATP) by the F1FO ATP synthase (Osellame et al., 2012). The balance of NADH to NAD+ in the mitochondria therefore reflects the balance of TCA cycle to ETC activity. ETC dysfunction causes increases in the NADH/NAD+ ratio and the production of potentially damaging reactive oxygen species (ROS) (Murphy, 2009). The cell’s antioxidant defences require the NADP pool to provide reducing equivalents for their maintenance, so the NADPH/NADP+ ratio must be maintained high (> 3) (Pollak et al., 2007). The redox state of the mitochondrial NAD pool and the relative abundance of NADPH are therefore key factors in the level of oxidative stress in a cell type.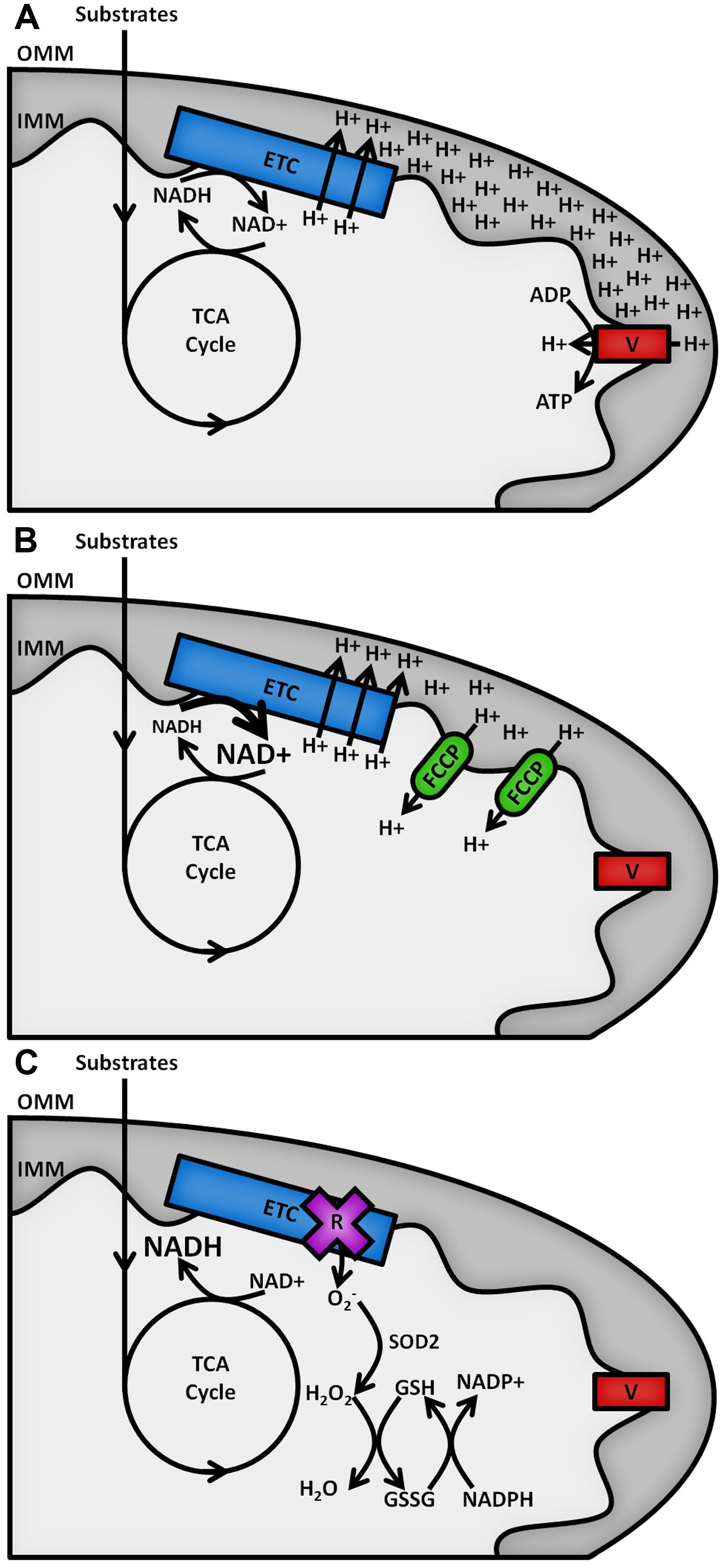
Figure 1. Schematic outline of mitochondrial NAD(P)H metabolism. Substrate oxidation in the TCA cycle passes electrons to NAD+, forming NADH. A. Under resting conditions, electrons carried by NADH are passed along the ETC, powering the pumping of protons from the mitochondrial matrix across the inner mitochondrial membrane (IMM) into the intermembrane space. The resulting proton gradient powers the production of ATP at complex V of the ETC (F1FO ATP synthase). B. Addition of an uncoupler such as carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) allows protons to leak back across the IMM, causing the rate of oxidation of NADH at the ETC to increase to restore the membrane gradient. C. Inhibition of the ETC by rotenone halts NADH oxidation and causes an increase in the production of superoxide (O2-), the proximal source of mitochondrial ROS. This is neutralised upon its conversion into water by the superoxide dismutase (SOD2) and glutathione (GSH/GSSG) antioxidant defence systems, maintained by NADPH.
Here, we describe protocols for the assessment of the mitochondrial NADH/NAD+ ratio and the NADPH/NADH balance that rely on the fluorescence of these cofactors when reduced. Their identical absorption and emission spectra leads the combined signal to be termed NAD(P)H (Blacker and Duchen, 2016). Measuring the change in NAD(P)H fluorescence using a confocal microscope following the application of an ETC uncoupler and inhibitor allows the mitochondrial NADH/NAD+ balance to be estimated (Duchen et al., 2003). To discriminate between the relative contributions of NADH and NADPH to the total signal, fluorescence lifetime imaging microscopy (FLIM) must be introduced (Blacker et al., 2014). These protocols describe, in further detail, methods used in Tosatto et al. (2016) to investigate the role of the selective channel responsible for mitochondrial calcium uptake, the mitochondrial calcium uniporter (MCU), in the progression of breast cancer. Basic understanding of confocal microscopy is assumed. For background, readers are directed to Pawley et al. (2012).
Materials and Reagents
- NuncTM cell culture treated EasYFlasksTM (75 cm2, filter closure) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 156499 )
- Falcon tubes (15 ml) (VWR, catalog number: 734-0451 )
- Tips
- Falcon tubes (50 ml) (VWR, catalog number: 734-0448 )
- Eppendorf tubes (1.5 ml) (VWR, catalog number: 211-2520 )
- NuncTM cell-culture treated multidishes (6 wells) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 140675 )
- Coverslips (22 mm, thickness No.1) (VWR, catalog number: 631-0158 )
- Syringe (20 ml, VWR, catalog number: 613-3921 )
- Filter steriliser (0.2 μm, VWR, catalog number: 514-0064 )
- MDA-MB-231 cells (ATCC, catalog number: HTB-26 )
- Advanced Dulbecco’s modified Eagle’s medium (DMEM) (500 ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 12491023 )
- Fetal bovine serum (FBS, heat inactivated, 50 ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 10500064 )
- GlutaMAXTM (5 ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061 )
- Penicillin-streptomycin (10,000 U ml-1, 5 ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Trypsin-EDTA (0.25%, with phenol red, 100 ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056 )
- FCCP (10 mg) (Sigma-Aldrich, catalog number: C2920 )
- Rotenone (5 g) (Sigma-Aldrich, catalog number: R8875 )
- Ethanol (1 L) (Sigma-Aldrich, catalog number: 02860 )
- Dulbecco’s modified Eagle’s medium powder (without glucose, L-glutamine, phenol red, sodium pyruvate and sodium bicarbonate) (Sigma-Aldrich, catalog number: D5030 )
- D-(+) glucose (100 g) (Sigma-Aldrich, catalog number: G8270 )
- Sodium pyruvate (25 g) (Sigma-Aldrich, catalog number: P2256 )
- HEPES (100 g) (Sigma-Aldrich, catalog number: H3375 )
- Sodium hydroxide solution (1 N, 1 L) (Sigma-Aldrich, catalog number: 71463 )
- Hydrochloric acid solution (1 N, 1 L) (Sigma-Aldrich, catalog number: 71763 )
- Routine cell culture medium (500 ml) (see Recipes)
- Live-cell imaging medium (50 ml) (see Recipes)
- ETC perturbations (see Recipes)
Equipment
- Incubator (37 °C, 5% CO2) (Thermo Fisher Scientific, Thermo ScientificTM, model: HERAcellTM 150i )
- Centrifuge with 15 ml Falcon tube capacity (Mega Star 1.6) (VWR, catalog number: 521-1749 )
- Haemocytometer (Neubauer) (VWR, catalog number: 630-1509 )
- Laser-scanning microscope (Zeiss, model: LSM510 )
- 40x magnification objective
- Blackout curtains, for room and covering FLIM microscope (see Figure 2)
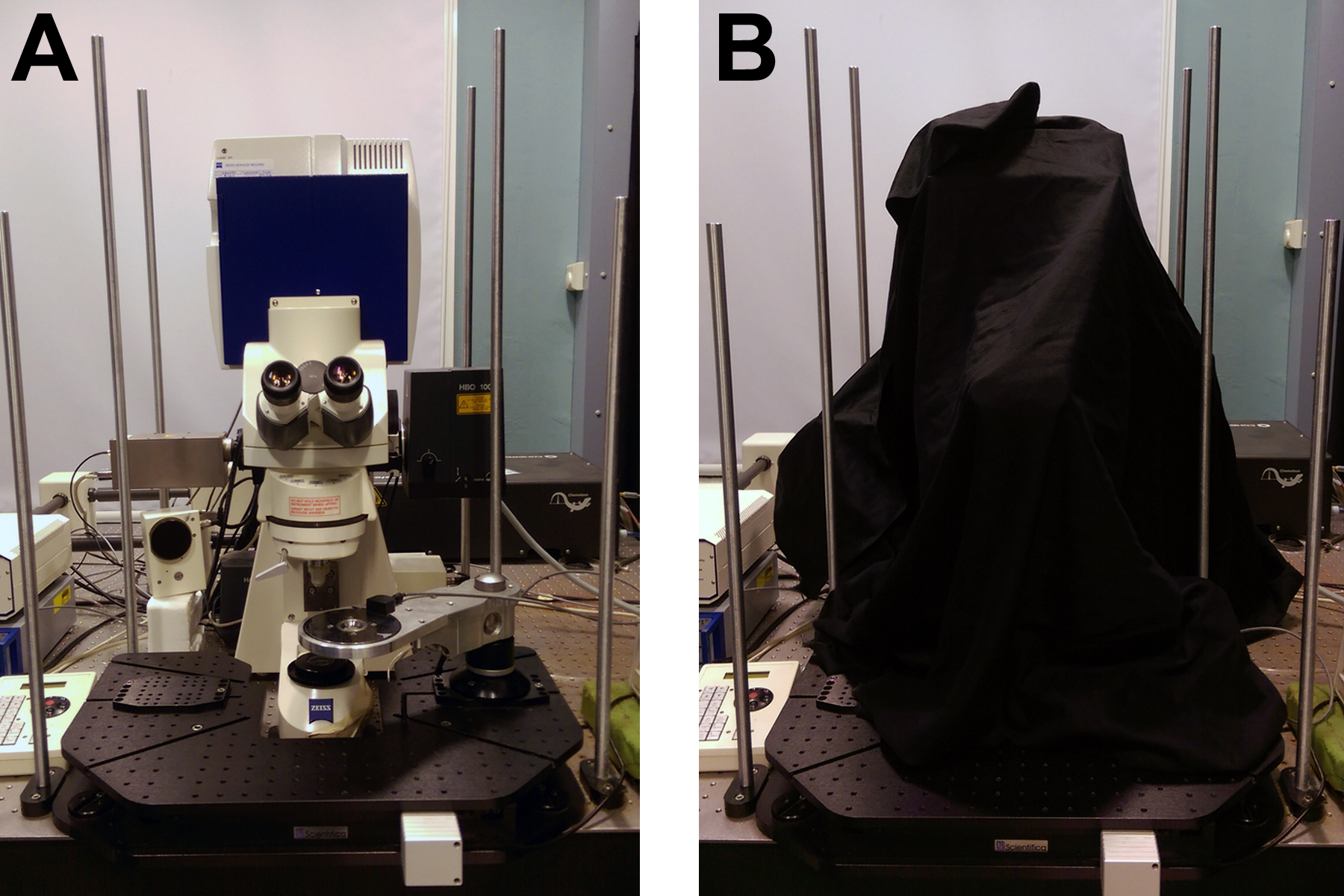
Figure 2. Equipment for high signal to noise FLIM. A. Zeiss LSM510 with Coherent Chameleon for two-photon excitation and Becker & Hickl HPM-100-40 detector and SPC830 counting electronics. B. Blackout curtains surrounding both the microscope room and the microscope itself act to ensure that background noise in the FLIM images is kept to a minimum. - Emission filters for NAD(P)H fluorescence (435-485 nm)
- For single-photon excitation:
- 351 nm laser source (Enterprise UV, Coherent)
- Long-pass dichroic (375 nm cutoff)
- Quartz microscope optics
- For two-photon excitation:
- Ti:sapphire laser modelocked at 720 nm (Chameleon Ultra, Coherent)
- Short pass dichroic (650 nm cutoff)
- Non-descanned detection
- For FLIM:
- Detector with single photon sensitivity (Becker & Hickl, model: HPM-100-40)
- Time-correlated single photon counting (TCSPC) electronics (Becker & Hickl, model: SPC-830)
- Pulsed excitation source (two-photon excitation by Ti:sapphire laser at 720 nm; Chameleon Ultra, Coherent)
- Heated microscope stage (custom built to hold imaging rings, see Figure 3)
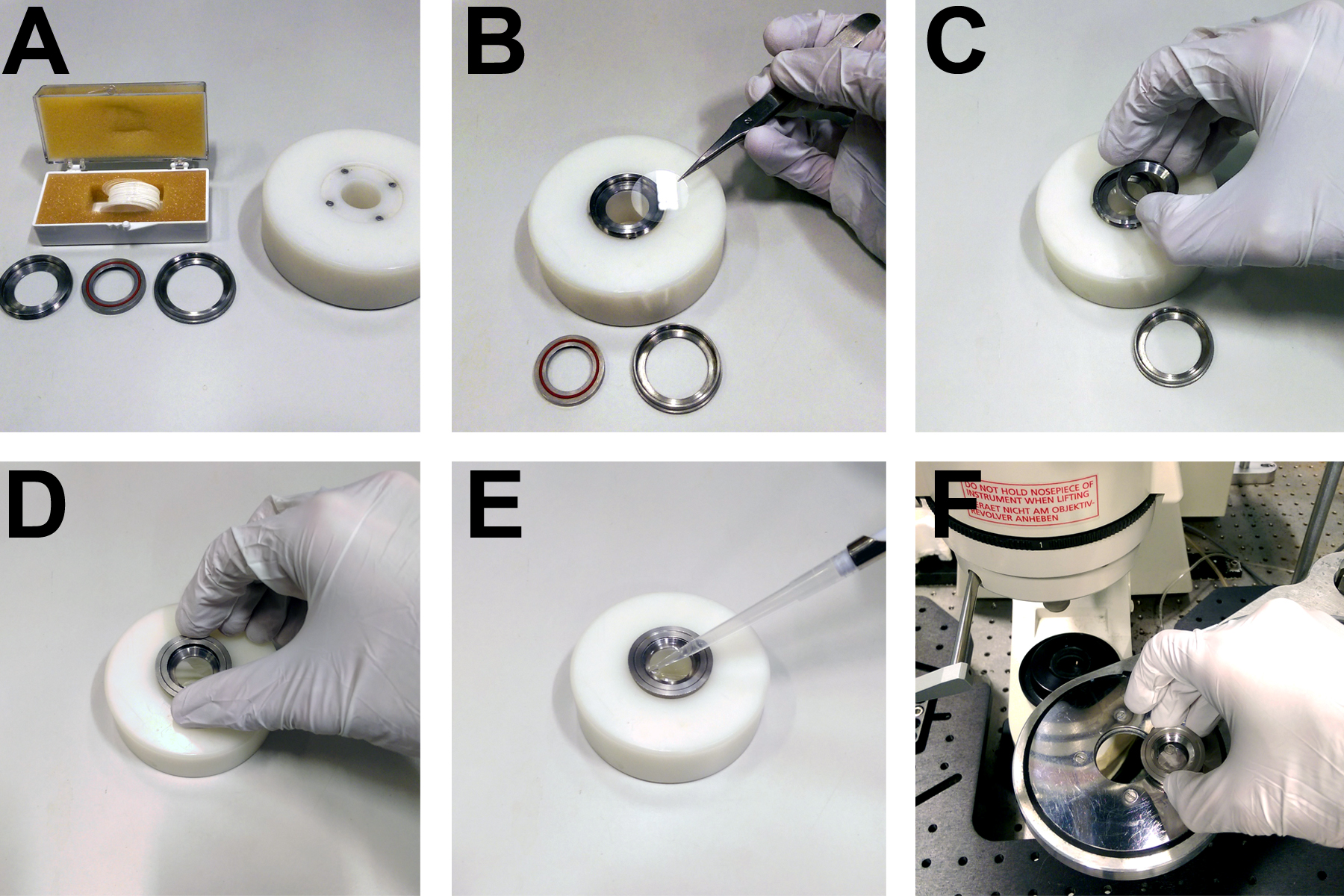
Figure 3. Apparatus for mounting coverslips on the microscope. A. Custom built rings and mounting block for securing 22 mm circular coverslips. B. Coverslips are placed directly onto the base of a metal ring. C. A concentric ring with a rubber seal is placed on top of the coverslip. D. A third ring is screwed onto the first two in order to form a watertight seal over the coverslip, allowing (E) the imaging buffer to be pipetted onto the cells. F. The imaging ring and cells then fit into a heated microscope stage.
- Straight-tip forceps (VWR, catalog number: 232-0094 )
- Imaging rings (custom built, see Figure 3)
- Inverted, phase-contrast, bench top microscope (VWR, catalog number: 630-2145 ; ZEISS, model: Primovert )
- Laboratory balance (0.1 mg resolution) (Mettler-Toledo, catalog number: 30029067 )
- pH meter (Mettler-Toledo International, catalog number: 30266626 )
- Tally counter (VWR, catalog number: 720-1984 )
Software
- Microscope control software (Zeiss ZEN)
- FLIM acquisition software (Becker & Hickl SPCM)
- FLIM fitting software (Becker & Hickl SPCImage)
- Image analysis software (NIH ImageJ)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Blacker, T. S., Berecz, T., Duchen, M. R. and Szabadkai, G. (2017). Assessment of Cellular Redox State Using NAD(P)H Fluorescence Intensity and Lifetime. Bio-protocol 7(2): e2105. DOI: 10.21769/BioProtoc.2105.
分类
癌症生物学 > 侵袭和转移 > 癌症治疗
癌症生物学 > 细胞能量学 > 细胞生物学试验 > 新陈代谢
细胞生物学 > 细胞成像 > 共聚焦显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link