- EN - English
- CN - 中文
Measuring Procaspase-8 and -10 Processing upon Apoptosis Induction
细胞凋亡诱导产生半胱天冬酶原-8和半胱天冬酶原-10的测定
发布: 2017年01月05日第7卷第1期 DOI: 10.21769/BioProtoc.2081 浏览次数: 10543
评审: Emilie BesnardAnonymous reviewer(s)

相关实验方案
细胞膜呈递CD40配体与可溶性激动剂激活CD40的体外共培养系统
Khalidah Ibraheem [...] Nikolaos T. Georgopoulos
2018年07月05日 9259 阅读
Abstract
Apoptosis or programmed cell death is important for multicellular organisms to keep cell homeostasis and for the clearance of mutated or infected cells. Apoptosis can be induced by intrinsic or extrinsic stimuli. The first event in extrinsic apoptosis is the formation of the Death-Inducing Signalling Complex (DISC), where the initiator caspases-8 and -10 are fully activated by several proteolytic cleavage steps and induce the caspase cascade leading to apoptotic cell death. Analysing the processing of procaspases-8 and -10 by Western blot is a commonly used method to study the induction of apoptosis by death receptor stimulation. To analyse procaspase-8 and -10 cleavage, cells are stimulated with a death ligand for different time intervals, lysed and subjected to Western blot analysis using anti-caspase-8 and anti-caspase-10 antibodies. This allows monitoring the caspase cleavage products and thereby induction of apoptosis.
Keywords: Caspase-8 (半胱天冬酶-8)Background
Caspases are proteases that are produced as inactive zymogens and are activated by proteolytic cleavage (Degterev et al., 2003). The activation of the caspase cascade is the most important event during apoptotic cell death, which induces the typical biochemical and morphological changes of the apoptotic cell. In contrast to inactive executioner procaspases, the initiator procaspases 8/9/10 have restricted proteolytic activity and become fully activated in high molecular weight complexes (Lavrik et al., 2005). Procaspase-9 is activated at the platform termed apoptosome during intrinsic apoptosis, while stimulation of the death receptors TNF-R1 (Tumor Necrosis Factor Receptor 1), CD95/Fas (Cluster of Differentiation 95), TRAIL-R1 (Tumor Necrosis Factor Related Apoptosis Inducing Ligand Receptor 1), TRAIL-R2 (Tumor Necrosis Factor Related Apoptosis Inducing Ligand Receptor 2) leads to the recruitment of procaspases-8 and -10 with adapter proteins to form the Death-Inducing Signalling Complex (DISC) during extrinsic apoptosis (Schleich et al., 2012). Here, caspases-8 and -10 enter close proximity and perform several intra- and inter-molecular cleavages. This processing results in the release of a small and a large subunit from the prodomain. These form the active heterotetramers p182p102 and p172p122 that triggers the caspase cascade leading to the demolition of the cell (see Figures 1A and 1B).
To verify apoptosis induction, it is important to show the activation of caspases. Checking the activation of the initiator procaspases 8 and 10, accompanied by their cleavage, gives main evidence for the induction of the extrinsic apoptosis via death receptors. The kinetics of procaspases-8 and -10 processing can be analysed by monitoring its cleavage steps by Western blot (see Figure 1C) and give information about the induction of apoptotic cell death after death receptor stimulation (Schleich et al., 2016). Depending on the part of the caspase that is recognized by the antibody, the intermediate products of the caspase containing this particular part can be analysed by western blot. Healthy, unstimulated cells only contain the unprocessed procaspases-8 and -10 (p55/53 and p59/55, respectively), but after stimulation the amount of procaspase decreases and the intermediate cleavage products (caspase-8 p43/41 or caspase-10 p47/43) and active subunits (caspase-8 p18) are enriched and can be detected by Western blot (Figure 1C). By performing time-dependent analysis, it is possible to follow the course of caspase cleavage including the enrichment of the cleaved forms (Schleich et al., 2016). This also allows to compare the time-dependent cleavage of caspases between several conditions.
To ensure getting the complete information on procaspase-8/-10 processing we have developed the protocol presented below. In contrast to a number of other protocols for Western blot analysis of procaspase-8/-10 processing, here we do not follow only a part of the Western blot of a specific molecular weight, e.g., by cutting the membrane, or use antibodies specific only to the active forms of the caspases. In this way, we can follow simultaneously several cleavage products of procaspases-8 and -10: proform, intermediate and final cleavage products. Only this way of measuring procaspase-8/-10 processing allows to escape from misjudgement on the efficiency of procaspase cleavage, e.g., in some studies only the proform of procaspases is followed and in case of a low stimulation strength and a weak caspase processing, the small differences in the decrease of the proform are not detected and might be misleading. Furthermore, another very important feature of our protocol is performing the measurement over different time intervals, which is also often neglected, and thus, the results might be misleading due to missing the key time points of processing, for example the appearance of active caspases, that are degraded fast. To the later point, there is a cell type and stimulation strength specificity with respect to the timing of procaspase-8/-10 processing and the corresponding time intervals have to be carefully selected in each particular case.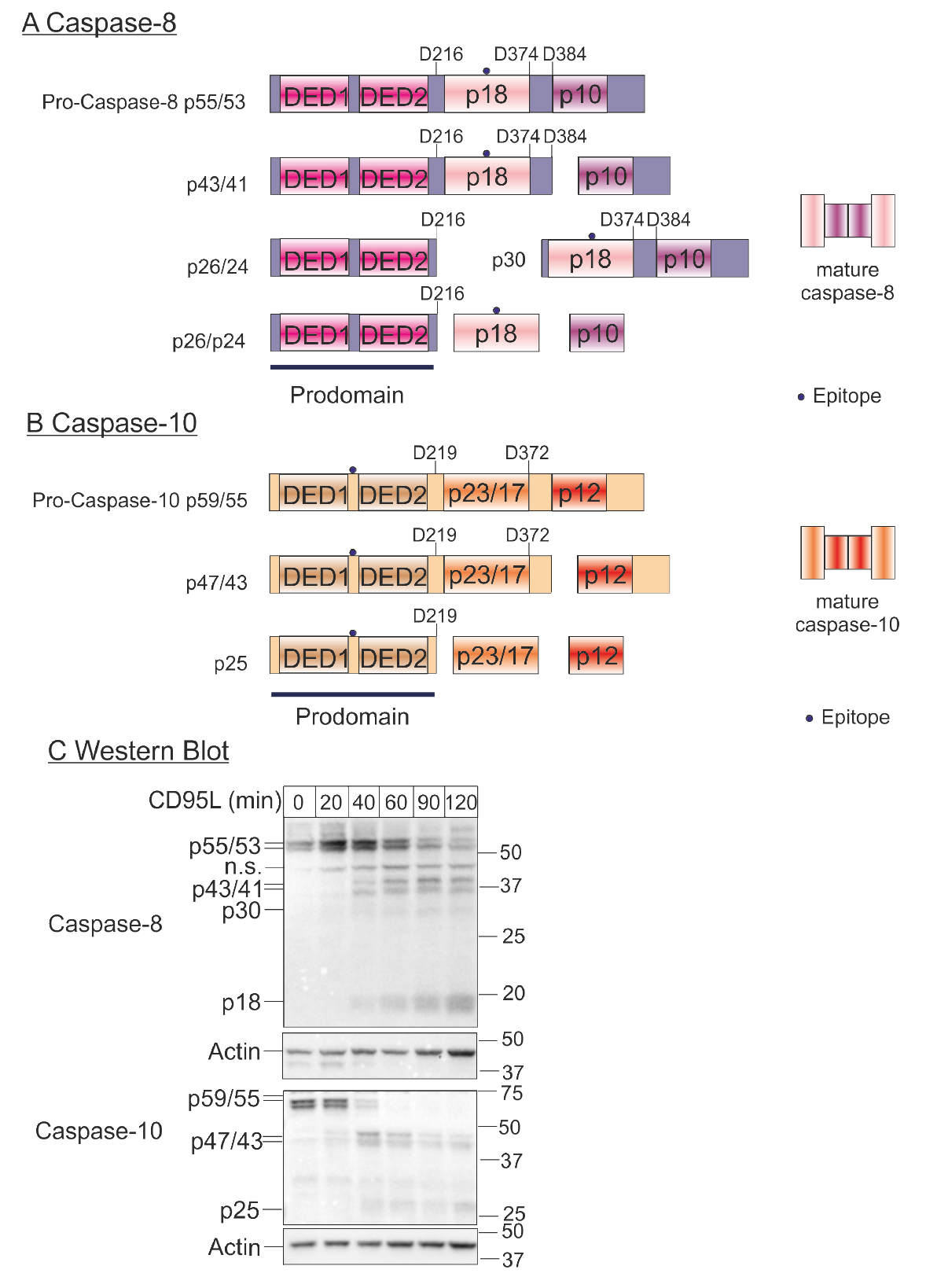
Figure 1. Kinetics of procaspase-8 and procaspase-10 processing. A. Scheme of caspase-8 processing. The cleavage at D216, D374 and D384 results in the release of the active subunits (p18 and p10). The point marks the region of the caspase that is recognized by the antibody (epitope). B. Scheme of caspase-10 processing. The cleavage at D219, D372 results in the release of the active subunits (p23/p17 and p12). The point marks the region of the caspase that is recognized by the antibody (epitope). C. Western blot: Hela-CD95 cells (Neumann et al., 2010) were stimulated with 250 ng/ml CD95L for the indicated periods of time. Samples were lysed, subjected to SDS-PAGE and analysed by Western blot for caspase-8 and caspase-10. Western blot for actin was used as loading control.
Materials and Reagents
- 6-well plates (with a surface suitable for your cells, e.g., SARSTEDT, catalog number: 83.3920 )
- Microcentrifuge tubes (e.g., VWR, catalog number: 89202.684 )
- Cuvettes (e.g., SARSTEDT, model: 67.742 )
- Blotting membrane and Whatman paper (e.g., Trans-Blot Turbo Transfer Pack Mini incl. 0.2 µm nitrocellulose and transfer buffer, Bio-Rad Laboratories, catalog number: 170-4158 )
- Human cells expressing caspase-8 and caspase-10, here: HeLa cells stably overexpressing CD95 (Neumann et al., 2010; DKMZ, catalog number: ACC 57 )
- Suitable medium for your cells (e.g., DMEM/Ham’s F12 including 10% fetal bovine serum and 1% penicillin/streptomycin)
- CD95L has been prepared as described in Fricker et al., 2010
- Bovine serum albumin (BSA) standard 2 mg/ml (Bio-Rad Laboratories, catalog number: 5000260 )
- SDS-poly acrylamide gel (e.g., Mini-PROTEAN TGX stain-free precast gels, 12%, Bio-Rad Laboratories, catalog number: 456-8046 )
- Protein assay dye reagent concentrate (Bio-Rad Laboratories, catalog number: 500-0006 )
- Protein standard (e.g., Precision plus Protein All Blue standards, Bio-Rad Laboratories, catalog number: 1610373 )
- Anti-caspase-8 antibody, clone C15 (final concentration 0.5-2 µg/ml, a kind gift of P. Krammer, DKFZ Heidelberg)
- Anti-caspase-10 antibody, clone 4C1 (dilution 1:1,000, final concentration 1 µg/ml, MEDICAL & BIOLOGICAL LABORATORIES, catalog number: MBL-M059-3 )
- Anti-actin antibody (dilution: 1:4,000, final concentration 0.125-0.2 µg/ml, Sigma-Aldrich, catalog number: A2103 )
- HRP-coupled isotype specific secondary antibodies (e.g., Santa Cruz Biotechnology, catalog numbers: sc-2060 , sc-2004 , sc-2062 )
- HRP-Substrate for Enhanced Luminescence, (e.g., Luminata Forte Western HRP Substrate, EMD Millipore, catalog number: WBLUF0500 )
- 10x PBS (Biochrom, catalog number: L1835 )
- cOmpleteTM protease inhibitor cocktail (PIC) (Roche Diagnostics, catalog number: 11 836 145 001 )
- Tris (AppliChem, catalog number: A2264 )
- Natrium chloride (NaCl) (Carl Roth, catalog number: P029.3 )
- EDTA (Carl Roth, catalog number: CN06.2 )
- Glycerol (Carl Roth, catalog number: 3783.1 )
- Triton (Carl Roth, catalog number: 3051.4 )
- Tween-20 (AppliChem, catalog number: A1389 )
- Milk powder (Carl Roth, catalog number: T145.5 )
- Sodium azide (Carl Roth, catalog number: K305.1 )
- β-mercaptoethanol (Carl Roth, catalog number: 4227.2 ).
- 10 x Tris/Glycine/SDS (Bio-Rad Laboratories, catalog number: 161-0723 )
- 4x Laemmli sample buffer (Bio-Rad Laboratories, catalog number: 161-0747 )
- 1x PBS (see Recipes)
- Protease inhibitor cocktail (PIC, see Recipes)
- Lysis buffer (see Recipes)
- 1 x Tris/Glycine/SDS (see Recipes)
- PBS-T (see Recipes)
- Blocking buffer (see Recipes)
- Primary antibody dilution (see Recipes)
- 4x Laemmli sample buffer (reducing, see Recipes)
Equipment
- CO2-incubator (e.g., Thermo Fisher Scientific, Thermo ScientificTM, model: BBD 6220 )
- Cell scraper (e.g., Orange Scientific, catalog number: 4460600N )
- Centrifuge (Eppendorf, model: 5418R )
- Photometer (Bio-Rad Laboratories, model: SmartSpec Plus Spectrophotometer )
- Electrophoresis chamber and power supply (Bio-Rad Laboratories, model: Mini-PROTEAN Tetra cell and PowerPac HC )
- Transfer system (Bio-Rad Laboratories, model: Trans-Blot Turbo Transfer System )
- Imager (Bio-Rad Laboratories, model: ChemiDoc XRS+ Imaging System )
Software
- Image Lab (Bio-Rad Laboratories)
Procedure
文章信息
版权信息
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Pietkiewicz, S., Wolfe, C., Buchbinder, J. H. and Lavrik, I. N. (2017). Measuring Procaspase-8 and -10 Processing upon Apoptosis Induction. Bio-protocol 7(1): e2081. DOI: 10.21769/BioProtoc.2081.
分类
癌症生物学 > 细胞死亡 > 细胞生物学试验 > 细胞凋亡
免疫学 > 免疫细胞功能 > 细胞毒性
分子生物学 > 蛋白质 > 表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link