- EN - English
- CN - 中文
Rapid Determination of Cellulose, Neutral Sugars, and Uronic Acids from Plant Cell Walls by One-step Two-step Hydrolysis and HPAEC-PAD
采用一步两步式水解法和HPAEC-PAD快速测定植物细胞壁的纤维素、中性糖和糖醛酸
(*contributed equally to this work) 发布: 2016年10月20日第6卷第20期 DOI: 10.21769/BioProtoc.1978 浏览次数: 18431
评审: Renate WeizbauerYingnan HouAnonymous reviewer(s)
Abstract
The plant cell wall is primarily composed of the polysaccharides cellulose, hemicellulose and pectin. The structural and compositional complexity of these components are important for determining cell wall function during plant growth. Moreover, cell wall structure defines a number of functional properties of plant-derived biomass, such as rheological properties of foods and feedstock suitability for the production of cellulosic biofuels. A typical characterization of cell wall chemistry in the molecular biology lab consists of a mild acid hydrolysis for the quantification of hemicellulose and pectin-derived monomers and a separate analysis of cellulose by the Updegraff method. We have adopted a streamlined ‘one-step two-step’ hydrolysis protocol that allows for the simultaneous determination of cellulose content, neutral sugars, and uronic acids by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) of paired samples. In our work, this protocol has largely replaced Updegraff cellulose quantification and hydrolysis with 2 M TFA for the determination of matrix polysaccharide composition at the micro scale.
Keywords: Cellulose (纤维素)Background
The protocol is based on paired analysis of samples hydrolyzed in 4% (w/v) sulfuric acid at 121 °C. One set of samples is first pretreated with 72% (w/w) sulfuric acid to swell cellulose and make it susceptible to dilute acid hydrolysis (Saeman hydrolysis in Figure 1; Saeman et al., 1945). The other set of samples are not subjected to this pretreatment, resulting in hydrolysis of non-crystalline matrix polysaccharides (Matrix hydrolysis in Figure 1). Comparison of the glucose recovered from each hydrolysis regime allows calculation of cellulose amount that is in good agreement with the more labor-intensive Updegraff (1969) protocol (Bauer and Ibáñez, 2014). In addition to glucose (Glc), other sugars derived from matrix polysaccharides can be quantified from the matrix hydrolysis samples (Gao et al., 2014). Thus, with relatively few manual manipulations, matrix monosaccharides and cellulose can be quantified from two hydrolysis samples and a total of four HPAEC-PAD experiments. Despite the number of chromatographic separations required by the protocol, the great reduction in ‘hands-on’ time required to prepare samples makes this technique well-suited to high throughput analyses. Although we have found that robust and reproducible analysis of rhamnose (Rha), arabinose (Ara), mannose (Man), and xylose (Xyl) requires multiple HPAEC-PAD runs, reports describing simultaneous quantification of all neutral and acidic sugars in a single run may allow further improvement of this protocol’s throughput (Zhang et al., 2012; Voiniciuc and Grünl, 2016). The steps of the protocol described here are outlined in Figure 1.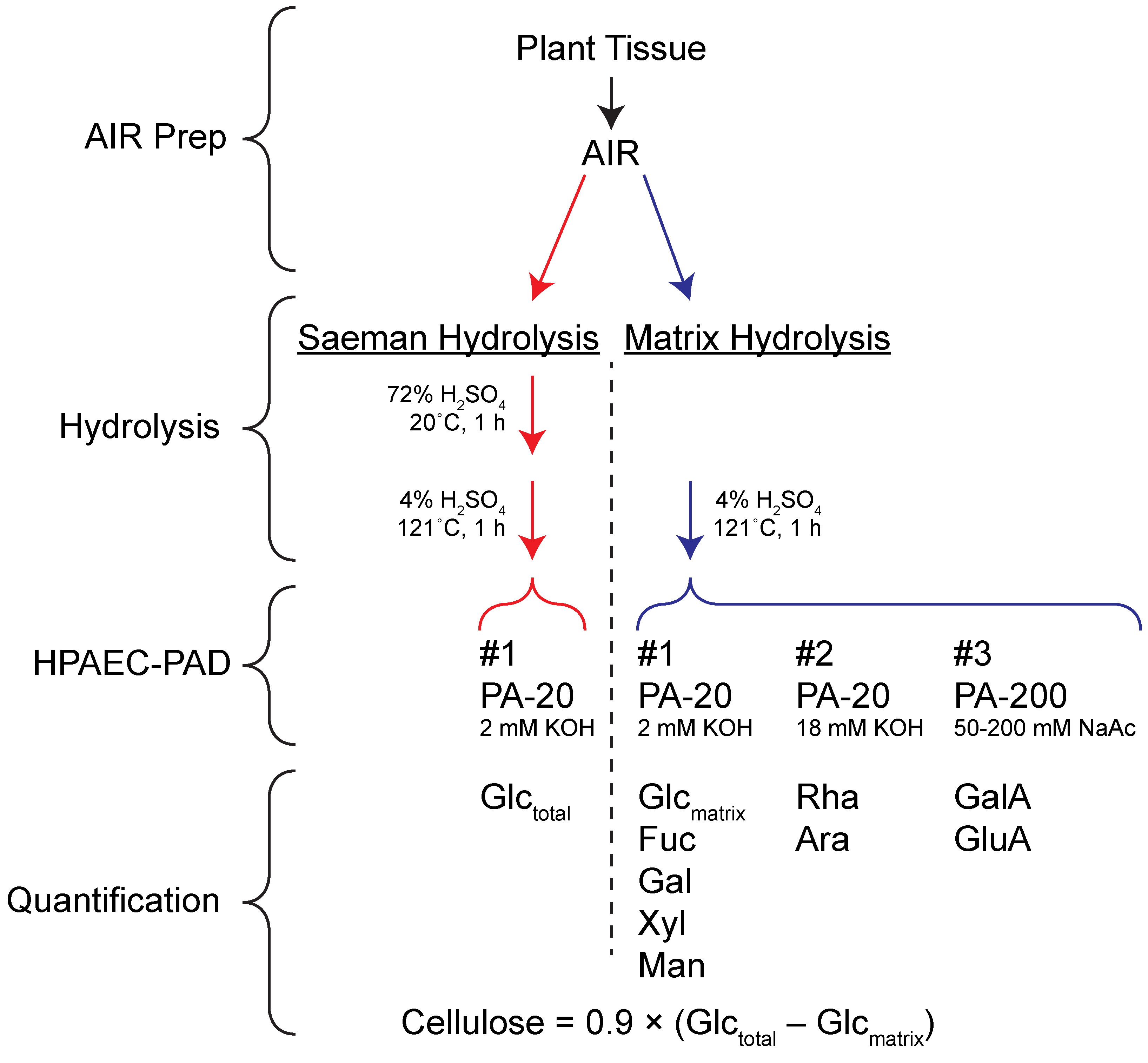
Figure 1. Protocol Overview. AIR = Alcohol insoluble residue.
Materials and Reagents
- 2 ml safe-lock microcentrifuge tubes (Eppendorf, catalog number: 022363352 )
- Aluminum foil
- 2 ml Sarstedt tubes with screw caps (SARSTEDT, catalog number: 72.694.007 )
- 2 ml screw cap autosampler vials (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: C4000-1W )*
- Autosampler vial caps with pre-slit septa (Phenomenex, catalog number: AR0-8977-13-B )*
- 5/32” Grinding Balls, 440C Stainless Steel, Treated (OPS Diagnostics, catalog number: GBSS 156-5000-01 )*
- Disposable anti-static polypropylene powder scoops (Cole-Parmer Instrument, catalog number: 06277-60 )
Note: This product has been discontinued. - Ethanol (Decon Labs, catalog number: V1016 )*
- Chloroform (Thermo Fisher Scientific, Fisher Scientific, catalog number: C607-4 )*
- Methanol (Thermo Fisher Scientific, Fisher Scientific, catalog number: A412P4 )*
- Acetone (Thermo Fisher Scientific, Fisher Scientific, catalog number: A184 )*
- 72% (w/w) sulfuric acid solution (RICCA Chemical, catalog number: R81916001A )*
- Ultrapure water (Milli-Q or equivalent)*
- 9 sugars:
L-fucose (Fuc) (Sigma-Aldrich, catalog number: F2252 )*
D-glucose (Glc) (Sigma-Aldrich, catalog number: G8270 )*
D-galactose (Gal) (Sigma-Aldrich, catalog number: G0750 )*
D-xylose (Xyl) (Sigma-Aldrich, catalog number: X1500 )*
D-mannose (Man) (Sigma-Aldrich, catalog number: M8574 )*
L-arabinose (Ara) (Sigma-Aldrich, catalog number: A3256 )*
L-rhamnose (Rha) (Sigma-Aldrich, catalog number: W373011 )*
D-galacturonic acid monohydrate (GalA) (Sigma-Aldrich, catalog number: 48280 )*
D-glucuronic acid (GluA) (Sigma-Aldrich, catalog number: G5269 )* - Sodium hydroxide solution (50%, w/w) (Thermo Fisher Scientific, Fisher Scientific, catalog number: SS254 )
- Sodium acetate, anhydrous (Sigma-Aldrich, catalog number: 71183 )
- Liquid nitrogen
*Note: These items can reliably be substituted with laboratory-grade equivalents from different vendors.
Equipment
- Freeze-dryer (Labconco, model: FreeZone 12 )* (optional)
- Magnet
- Aspirator
- Metal spatula
- Microcentrifuge (Eppendorf, model: 5417R )*
- Autoclave-compatible rack
- Autosampler
- Microbalance (Mettler Toledo, model: XS105 )*
- Ball mill (Retsch, model: MM 400 )*
- Dionex ICS-5000 HPAEC-PAD system, optionally equipped with an eluent generator (Thermo Fisher Scientific, Fisher Scientific, model: ICS-5000+ SYSTEM )
- CarboPac PA-20 Analytical column, 3 x 150 mm (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 060142 )
- CarboPac PA-20 Guard column, 3 x 30 mm (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 060144 )
- CarboPac PA-200 Analytical column, 3 x 250 mm (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 062896 )
- CarboPac PA-200 Guard column, 3 x 50 mm (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 062895 )
*Note: These items can reliably be substituted with laboratory-grade equivalents from different vendors.
Software
- Chromeleon 7 (Thermo Fisher Scientific)
- Microsoft Excel
Procedure
文章信息
版权信息
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yeats, T., Vellosillo, T., Sorek, N., Ibáñez, A. B. and Bauer, S. (2016). Rapid Determination of Cellulose, Neutral Sugars, and Uronic Acids from Plant Cell Walls by One-step Two-step Hydrolysis and HPAEC-PAD. Bio-protocol 6(20): e1978. DOI: 10.21769/BioProtoc.1978.
- Yeats, T. H., Sorek, H., Wemmer, D. E. and Somerville, C. R. (2016). Cellulose deficiency is enhanced on hyper accumulation of sucrose by a H+-coupled sucrose symporter. Plant Physiol 171(1): 110-124.
分类
植物科学 > 植物生物化学 > 糖类
生物化学 > 糖类 > 纤维素
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link