- EN - English
- CN - 中文
Extraction and Assays of ADP-glucose Pyrophosphorylase, Soluble Starch Synthase and Granule Bound Starch Synthase from Wheat (Triticum aestivum L.) Grains
从小麦谷粒提取和检测ADP-葡萄糖焦磷酸化酶、可溶性淀粉合成酶和淀粉粒束缚淀粉合成酶
发布: 2016年09月20日第6卷第18期 DOI: 10.21769/BioProtoc.1929 浏览次数: 14555
评审: Samik BhattacharyaAnonymous reviewer(s)

相关实验方案
基于活性蛋白质组学和二维聚丙烯酰胺凝胶电泳(2D-PAGE)鉴定拟南芥细胞间隙液中的靶蛋白酶
Sayaka Matsui and Yoshikatsu Matsubayashi
2025年03月05日 1968 阅读
Abstract
Starch biosynthesis in plants involves a network of enzymes of which adenosine-5’-diphosphoglucose (ADP-glucose) pyrophosphorylase (AGPase, E.C. 2.7.7.27), and soluble and granule bound starch synthases (SSS and GBSS, E.C. 2.4.1.21) play central roles. Here, we outline the protocol for extraction and assay of these enzymes in developing grains of wheat (Triticum aestivum L.). The principle of the assays outlined is based on a coupling enzymatic reactions where the product of the initial reaction is used as a substrate for subsequent reactions in order to generate NADPH, which can be measured easily by spectrophotometer. This protocol does not need expensive labelled chemicals and can be carried out using equipment commonly found in a biochemical laboratory. We applied this protocol to study the dynamics of AGPase, SSS and GBSS activity in developing wheat grains at different time points after anthesis.
Keywords: Enzyme (酶)Background
Starch is a carbohydrate polymer made up of amylose, a linear glucan polymer composed of α-1,4-linked glucose molecules, and amylopectin, another glucan polymer composed of α-1,4-linked glucose molecules branched by α-1,6-glycosidic bonds. The enzyme adenosine-5’-diphosphoglucose (ADP-glucose) pyrophosphorylase (AGPase, E.C. 2.7.7.27) catalyzes the first committed step of starch synthesis, converting glucose-1-phosphate and ATP to ADP-glucose and inorganic pyrophosphate (PPi). ADP-glucose is subsequently used by soluble starch synthases (SSS) and granule bound starch synthases (GBSS) (E.C. 2.4.1.21), and starch branching enzymes to elongate and branch the glucan chains of the starch granule.
Initially, AGPase and starch synthase assays were carried out using 14C- and 32P-labelled ADP-glucose (Ghosh and Price, 1966; Vos-Scheperkeuter et al., 1986), which requires the use of expensive chemicals as well as specialized equipment to work with labelled compounds. Here, we outline the method adopted and applied for extracting and assaying AGPase, SSS and GBSS activity in developing wheat (Triticum aestivum L.) grains (Mukherjee et al., 2015). Our protocol is based on the methods reported previously by Nakamura et al. (1989) for measuring AGPase and SSS activities in the endosperm of developing rice grains, and by Schaffer and Petreikov (1997) for measuring the activities of SSS and GBSS in tomato fruits. The method is based on coupling enzymatic reactions where the product of the initial reaction is used as a substrate for subsequent reactions in order to generate NADPH, which can be easily measured by spectrophotometers. The protocol can be carried out using the equipment commonly found in biochemical laboratories and does not require the use of labeled compounds.
Because this protocol is an adoption of the methods developed for different tissues of other plant species (endosperm of rice grain and tomato fruit), the reaction mixture composition for all enzymes was optimized for reaction buffer pH and substrate concentration so that enzyme activity was within the linear phase with respect to incubation time and protein concentration. The amounts of enzyme preparation added (PGM, pyruvate kinase, hexokinase, G6PDH) have been adjusted to achieve completion of coupling reaction in expected time frame. This protocol can be used for studying the activities of AGPase, SSS and GBSS in other tissues of wheat as well as in different tissues of other plant species; however, optimization of reaction buffer pH and substrate concentrations is required.
The principle of AGPase assay is presented in Figure 1. AGPase present in plant tissue extract catalyzes the conversion of ADP-glucose and PPi into glucose-1-phosphate and ATP. After the first reaction is stopped by boiling, phosphoglucomutase (PGM) is added to the reaction mixture; PGM converts quantitatively glucose-1-phosphate into glucose-6-phosphate. Subsequently, glucose-6-phosphate dehydrogenase (G6PDH) is added; G6PDH converts glucose-6-phosphate in the presence of NADP into 6-phosphogluconic acid. At the same time, NADP is converted into NADPH and the quantity of NADPH formed is equivalent to the quantity of glucose-6-phosphate oxidized. By measuring NADPH concentration spectrophotometrically at 340 nm, we can quantify the amount of ADP-glucose degraded by AGPase activity.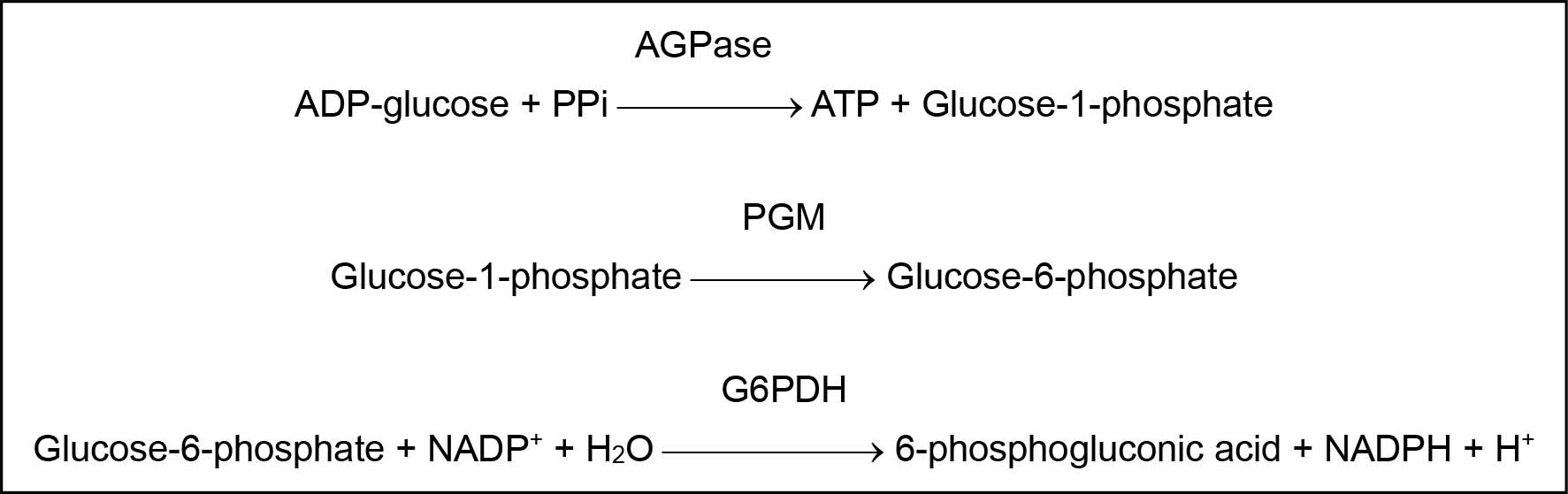
Figure 1. Principle for ADP-glucose pyrophosphorylase (AGPase) assay
The principle of SSS/GBSS assay is presented in Figure 2. Starch synthases present in plant tissue extract (SSS) or in starch granule suspension (GBSS) catalyze the conversion of ADP-glucose into ADP coupled with the elongation of amylopectin primer for one glucose residue. Pyruvate kinase added to the reaction mixture after the first reaction is stopped by boiling converts quantitatively ADP into ATP in the presence of PEP. Subsequently, addition of glucose together with hexokinase leads to the conversion of ATP into glucose-6-phosphate. In the next step of the assay, G6PDH converts glucose-6-phosphate in the presence of NADP into 6-phosphogluconic acid; NADP is converted into NADPH at the same time and the quantity of NADPH formed is equivalent to the quantity of glucose-6-phosphate oxidized. By measuring NADPH concentration spectrophotometrically at 340 nm, we can quantify the amount of ADP-glucose (at the first step of the analysis) degraded by SSS/GBSS activity.
Figure 2. Principle for soluble and granule bound starch synthases (SSS and GBSS) assay
Materials and Reagents
- Chemicals
- Common chemicals, buffers and kits
- Protein assay kit (Bio-Rad Laboratories, catalog number: 5000002 )
- 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Thermo Fisher Scientific, Fisher Scientific, catalog number: BP310 )
- Sodium hydroxide (NaOH) (Thermo Fisher Scientific, Fisher Scientific, catalog number: BP359 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2670 )
- Ethylenediaminetetraacetic acid (EDTA), disodium salt dihydrate (Na2EDTA·2H2O) (Sigma-Aldrich, catalog number: E1644 )
- DL-dithiothreitol (DTT) (Sigma-Aldrich, catalog number: D0632 )
- Polyvinylpyrrolidone (average molecular weight 10,000) (Sigma-Aldrich, catalog number: PVP10 )
- Potassium chloride (KCl) (Thermo Fisher Scientific, Fisher Scientific, catalog number: BP366 )
- Tetrasodium pyrophosphate (PPi) decahydrate (Na4P2O7·10H2O) (Sigma-Aldrich, catalog number: S6422 )
- Protein assay kit (Bio-Rad Laboratories, catalog number: 5000002 )
- Substrates and cofactors
- Adenosine-5’-diphosphoglucose (ADP-glucose), disodium salt (Sigma-Aldrich, catalog number: A0627 )
- Amylopectin from maize (Sigma-Aldrich, catalog number: 10120 )
- β-Nicotinamide adenine dinucleotide phosphate (NADP) sodium salt hydrate (Sigma-Aldrich, catalog number: N0505 )
- Phospho(enol)pyruvic acid (PEP) monosodium salt hydrate (Sigma-Aldrich, catalog number: P0564 )
- Adenosine-5’-diphosphoglucose (ADP-glucose), disodium salt (Sigma-Aldrich, catalog number: A0627 )
- Enzymes
- Hexokinase (E.C. 2.7.1.1) from Saccharomyces cerevisiae (Sigma-Aldrich, catalog number: H5000 )
- Glucose-6-phosphate dehydrogenase (G6PDH, E.C. 1.1.1.49) from Saccharomyces cerevisiae (Sigma-Aldrich, catalog number: G7877 )
- Phosphoglucomutase (PGM, E.C. 5.4.2.2) from rabbit muscle (Sigma-Aldrich, catalog number: P3397 )
- Pyruvate kinase (E. C. 2.7.1.40) from rabbit muscle (Sigma-Aldrich, catalog number: P1506 )
- Hexokinase (E.C. 2.7.1.1) from Saccharomyces cerevisiae (Sigma-Aldrich, catalog number: H5000 )
- Stock solutions (see Recipes)
- 20 mM ADP-glucose
- 10 mM NADP
- 20 mM PEP
- 20 mM ADP-glucose
- Common chemicals, buffers and kits
- Enzymatic activities reaction mixtures
- Extraction buffer (see Recipes, section 1)
- AGPase activity reaction mixture (see Recipes, section 3a)
- Starch synthase activity reaction mixture (see Recipes, section 3b)
- Solution 1 for starch synthase activity assay (Pyruvate kinase reaction mixture) (see Recipes, section 3c)
- Solution 2 for starch synthase activity assay (Glucose-6-phosphate dehydrogenase reaction mixture) (see Recipes, section 3d)
- Extraction buffer (see Recipes, section 1)
- Plant material
- Grains of common wheat (Triticum aestivum L.)
- Grains of common wheat (Triticum aestivum L.)
- Other materials
- Eppendorf tubes (1.5 and 2.0 ml)
- Falcon tubes (15 ml)
- Liquid nitrogen
- Microcentrifuge tube locker (for example, Sigma-Aldrich, catalog number: Z708372 , or Sorenson Bioscience MCT LidLockTM, catalog number: 11870 )
Note: Product Z708372 has been discontinued. - Miracloth (Merck Millipore, catalog number: 475855 )
- Eppendorf tubes (1.5 and 2.0 ml)
Equipment
- Porcelain mortar and pestle
- Analytical balance (capacity - 100 g or higher, resolution - 0.001 g or better)
- Digital heat block or water bath suitable for 30 °C and digital heat block or water bath suitable for 100 °C
Note: For some steps of the analysis, immediate transfer from 30 °C to 100 °C is necessary. This is why two heating devices are necessary simultaneously for the protocol. - Refrigerating ultracentrifuge with appropriate rotor suitable for spinning of 15 ml Falcon tube and 1.5-2 ml Eppendorf tubes at up to 12,000 x g
- Ultraviolet (UV)/Visible spectrophotometer equipped with heated cell holder suitable for maintaining cuvette temperature at 30 °C. We used GE Healthcare/Amersham Bioscience Ultrospec 3100 Pro UV/Visible spectrophotometer (Biochrom Ltd, Cambridge, UK).
Procedure
文章信息
版权信息
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Kulichikhin, K., Mukherjee, S. and Ayele, B. T. (2016). Extraction and Assays of ADP-glucose Pyrophosphorylase, Soluble Starch Synthase and Granule Bound Starch Synthase from Wheat (Triticum aestivum L.) Grains . Bio-protocol 6(18): e1929. DOI: 10.21769/BioProtoc.1929.
分类
植物科学 > 植物生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 分离和纯化
植物科学 > 植物生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link