- EN - English
- CN - 中文
Preparation of Recombinant Galectin-3 for Cancer Studies
用于癌症研究的重组半乳糖凝集素-3的制备
发布: 2016年01月05日第6卷第1期 DOI: 10.21769/BioProtoc.1696 浏览次数: 9346
评审: HongLok LungClara Lubeseder-MartellatoAnonymous reviewer(s)

相关实验方案
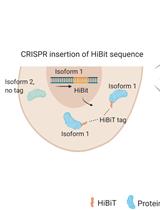
通过CRISPR-Cas9介导的HiBiT标签对高度同源蛋白水平进行异构体特异性半定量测定
Kristina Seiler [...] Mario P. Tschan
2023年07月20日 2519 阅读
Abstract
Galectin-3 is a member of a class of proteins termed Galectins, characterized by their ability to bind glycans containing β-galactose (Cummings and Liu, 2009). Galectin-3 binds preferentially to proteoglycans terminating with N-acetyllactosamine (LacNAc) chains (i.e., tandem repeats of galactose) (Newlaczyl and Yu, 2011). Galectin-3 is unique among the galectins in its chimeric structure. It shares a conserved carbohydrate recognition domain (CRD) with the other galectins, but has a long amino-terminal tail that is thought to be involved in protein aggregation. It can also form homodimers through its CRD (Cummings and Liu, 2009). Galectin-3 has been found to have diverse functions in tumorigenesis including: signaling, apoptosis inhibition, immune suppression, cell growth, and metastasis among others. Galectin-3 is frequently upregulated in cancers (Nangia-Makker et al., 2008). Its function largely depends on its expression and localization properties (Newlaczyl and Yu, 2011). Because of its many roles in cancer-associated processes, establishing a method for Galectin-3 production is valuable for further study of its functions in cancer. Here, we describe how Galectin-3 purification was achieved by cloning of the human Galectin-3 gene into pGEX-2T vector containing the gene for glutathione-S-transferase (GST) upstream of its cloning site. The Galectin-3 gene was cloned into this vector via restriction digests of both the plasmid and the Galectin-3 gene by restriction enzymes BamHI and EcoRI, followed by ligation of the two fragments. The resulting plasmid was then used to transform BL21, an Escherichia coli (E. coli) strain specialized for protein expression. Finally, we discuss how the GST fusion protein was isolated and the recombinant Galectin-3 protein was further purified from the GST.
Keywords: Cancer (癌症)Part I. Protocol for GST-Gal3 purification (Harper and Speicher, 2011)
Materials and Reagents
- Microcentrifuge tubes (Denville Posi-Click Tubes, catalog number: C-2170 )
- 15 ml conical tubes (Thermo Fisher Scientific, Corning CentriStar, catalog number: 05-538-59A )
- 10 ml serological pipettes (Thermo Fisher Scientific, Corning Costar, catalog number: 4488 )
- One Shot® BL21 StarTM (DE3) Chemically Competent E. coli (Thermo Fisher Scientific, catalog number: C6010-03 )
- pGEX 2T-Gal3 vector (kindly provided by Dr. W. Stallcup, UCSD, CA)
- L-broth (Thermo Fisher Scientific, catalog number: BP1426-2 )
- Glutathione-Agarose Beads (Thermo Fisher Scientific, PierceTM, catalog number: 16100 )
- Protease Inhibitor Tablets, EDTA-free (Thermo Fisher Scientific, PierceTM, catalog number: 88665 )
- Ice
- Purified Water
- 10x PBS, diluted to 1x (Thermo Fisher Scientific, Hyclone, catalog number: BP3994 )
- Isopropyl-beta-D-thiogalactopyranoside (IPTG) (Gold Biotechnology, catalog number: 12481C25 )
- Tris-Cl (pH 7.5)
- 150 mM NaCl
- 0.75% CHAPS powder (AG. Scientific, catalog number: C-1019 )
- 0.75% CHAPS buffer (see Recipes)
Equipment
- Microfuge (Beckman Coulter, model: Microfuge 16 )
- 37 °C shaker (FormaOrbital Shaker)
- Shaker (GMI, Lab Companion, model: SK-300 Shaker )
- Rotating Platform (24 rpm) (Benchmark model: MiniMixer )
- Vortex (Thermo Fisher Scientific, catalog number: 12-812 )
Note: Currently, it is “LabX, catalog number: 12-812”. - Centrifuge (Beckman Coulter, model: Avanti J-20 XP )
- Centrifuge rotor (Beckman Coulter, model: JLA-16.250 )
- Centrifuge (Beckman Coulter, model: Allegra 25R )
- Centrifuge rotor (Beckman Coulter, model: TS-5.1-500 )
- 250 ml plastic centrifuge bottles (Sigma-Aldrich, Nalgene®, model: B1033 )
- 2 L flask (Corning, Pyrex®, model: No. 4980 )
- 10 ml serological pipettes (Corning, Costar®, catalog number: 4488 )
Procedure
文章信息
版权信息
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Tyler, K., Lee, S. and Van Meir, E. G. (2016). Preparation of Recombinant Galectin-3 for Cancer Studies. Bio-protocol 6(1): e1696. DOI: 10.21769/BioProtoc.1696.
分类
癌症生物学 > 通用技术 > 生物化学试验 > 蛋白质分析
癌症生物学 > 通用技术 > 肿瘤微环境 > 细胞外基质
生物化学 > 蛋白质 > 表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link