- EN - English
- CN - 中文
Pyridine Hemochromagen Assay for Determining the Concentration of Heme in Purified Protein Solutions
吡啶血色原法测定纯化蛋白溶液中血红素的浓度
发布: 2015年09月20日第5卷第18期 DOI: 10.21769/BioProtoc.1594 浏览次数: 33548
Abstract
Heme is a common cofactor in proteins, found in hemoglobin, myoglobin, cytochrome P450, DGCR8, and nitric oxide synthase, among others. This protocol describes a method for quantifying heme that works best in purified protein samples. This protocol might be used to, for example, determine whether a given heme-binding protein is fully occupied by heme, thus allowing correlation of heme content with activity. This requires the absolute heme concentration and an accurate protein concentration. Another use is to determine the extinction coefficients of a heme-bound protein. This assay is fast, easy, and reproducible if done correctly.
Keywords: heme (血红素)Background
G.G. Stokes was the first to prepare what we now refer to as hemochromagen. As early as 1863, he was monitoring changes in the hemoglobin absorbance spectrum upon reduction of the heme to the Fe(II) form Stokes (1863). Stokes had reduced blood in the presence of ammonia; what he was seeing were the intense α and β peaks of Fe(II) heme b from hemoglobin in complex with ammonia. Later authors (Anson and Mirsky, 1928) were able to show that the hemochromagen, as it had been called by Christian Bohr (Edsall, 1972) , was heme in complex with some nitrogenous ligand. It can be formed as well by simply reducing myoglobin under denaturing conditions, in which case histidines serve as the axial ligands. Hill demonstrated that the pyridine hemochromagen is formed from the nitrogen of two pyridine molecules binding to the axial position of the reduced heme (Hill, 1926; Hill, 1929); this was confirmed by Smith (1959) and Gallagher and Elliot (Gallagher et al., 1965; Gallagher et al., 1968).
With advances in spectroscopic techniques, later workers were able to use hemochromagen for an important analytical purpose (Hill, 1929). The regularity of the α peak of reduced pyridine hemochromagen, its high extinction coefficient, and the fact that it follows Beer's Law over a wide range allows its use for determining the total heme composition of a sample. De Duve (1948) published one of the first protocols for this, and determined the extinction coefficient (ε) at 557 nm to be 32 mM-1 cm-1. The method used relied on gravimetric determination of the standard heme samples, which could lead to an underestimate of the true heme concentration if the sample is impure. Paul et al. (1953) re-determined the extinction coefficient for pyridine hemochromogen from recrystallized heme b and myoglobin, using the iron content as an internal control. They found a value (34.7 mM-1 cm-1) significantly higher than the previous value of 32 mM-1 cm-1, representing a difference of roughly 9%. Both values have been in use comparatively recently: e.g. (Scott and Lecomte, 2000; Fushitani and Riggs, 1988; Miyoshi et al., 1997; Yachie et al., 1999; Lee et al., 2000; Huche et al., 2006), which use 32 mM-1 cm-1, and (Sono et al., 1984; Senturia et al., 2012; Sinclair et al., 2001; Berry and Trumpower, 1987), which use 34.7 mM-1 cm-1. In this assay we have taken the value given by Paul et al. (1953) to be most accurate.
[Principle of action] The heme-containing protein solution is first mixed with Solution I (see Recipes) containing pyridine, NaOH and potassium ferricyanide. Pyridine serves as a ligand for heme in the Fe(II) state. NaOH keeps sodium dithionite stable. Potassium ferricyanide ensures that all dissolved heme is in the Fe(III) state at first. Then, excess sodium dithionite in solution is added to reduce the heme from Fe(III) to Fe(II). Another option is to use a pipette tip to add a few grains of solid dithionite to the cuvette, and allow it to dissolve. Recipes similar to the current one have been recommended by Sinclair et al. (2001); Berry and Trumpower (1987); Antonini and Brunori (1971). The concentration of heme in the sample can be determined from the absorbance of the reduced sample; the extinction coefficient for reduced pyridine hemochromagen is 34.7 mM-1 cm-1 at 557 nm for heme b. It can also be done using the difference spectrum between the reduced and oxidized samples. Don't forget, in either case, to take your dilution factor into consideration. This protocol is written for heme b; however, other types of heme form hemochromagens as well and can be quantified using the same technique. See Berry and Trumpower (1987) or Table 1 for extinction coefficients for hemes a and c.
Materials and Reagents
- Heme-containing sample
Note: The concentration of heme in your stock solution should be at least 10 μM and not much greater than 80 μM. Dithiothreitol (DTT) and other reducing agents can reduce potassium ferricyanide and may interfere with the oxidized sample, but not with the reduced sample. Any common biological buffer should be compatible with this procedure so long as it has no significant absorbance in the 500 nm to 600 nm range and does not form a complex with heme, as should be the case with all Good's buffers. We have personally used phosphate, tris, HEPES, EPPS, MES, and CHES without issue. - 0.5 M NaOH
- Pyridine (Sigma-Aldrich, catalog number: 360570 )
- Potassium ferricyanide(III) (Sigma-Aldrich, catalog number: 702587 )
- Sodium dithionite (Sigma-Aldrich, catalog number: 157953-5 G )
- Deionized water
- 0.2 M NaOH, 40% (v/v) pyridine, 500 μM potassium ferricyanide (see Recipes)
- 0.1 M potassium ferricyanide (K3[Fe(CN)6]) (see Recipes)
- 0.5 M sodium dithionite in 0.5 M NaOH (see Recipes)
Equipment
- Spectrophotometer with a bandwidth ≤ 2 nm
- Fume hood
- Quartz or glass cuvette, 1 cm length
- Pipettes
Note: It is generally good to have a spectrophotometer capable of relatively low spectral bandwidth (SBW) in order to get highly accurate measurements. It is suggested in the literature that the ratio of SBW to the natural bandwidth (NBW) should be 0.1 or lower in order to get an error of less than 0.5% (Surles and Erickson, 1974; Brodersen, 1954). The α band of reduced pyridine hemochromagen has a NBW of around 20 nm, hence the recommendation of 2 nm or less for SBW. Check with your manufacturer if you are unsure of your SBW. Most modern spectrophotometers have SBW less than 4 nm, corresponding to an error of less than 2%. It is also important to note that this error is not random, but results in an underestimate of the true absorbance; having a higher SBW/NBW ratio leads to a 'flattening' of absorbance peaks. If possible, use a scanning spectrophotometer. This allows you to verify that your spectrum looks similar to the spectrum shown in Figure 1.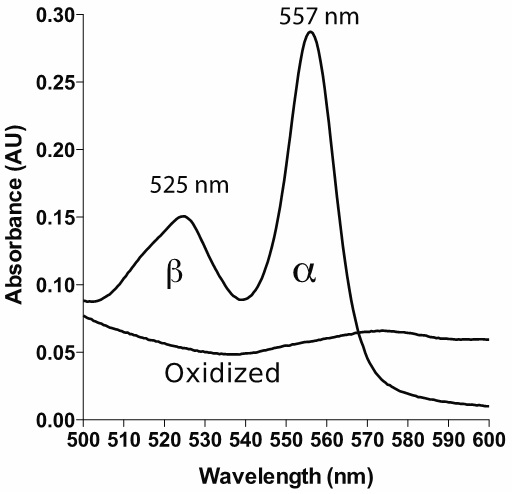
Figure 1. Example spectrum of reduced and oxidized pyridine hemochromagen (heme b). The bandwidth is set to 1 nm, with data interval 1 nm.
Procedure
文章信息
版权信息
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Barr, I. and Guo, F. (2015). Pyridine Hemochromagen Assay for Determining the Concentration of Heme in Purified Protein Solutions. Bio-protocol 5(18): e1594. DOI: 10.21769/BioProtoc.1594.
分类
生物化学 > 其它化合物 > 血红素
生物化学 > 蛋白质 > 相互作用 > 蛋白质-配体相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link