- EN - English
- CN - 中文
Electron Paramagnetic Resonance (EPR) Spectroscopy to Detect Reactive Oxygen Species in Staphylococcus aureus
电子顺磁共振(EPR)光谱法检测金黄色葡萄球菌中的活性氧类物质
发布: 2015年09月05日第5卷第17期 DOI: 10.21769/BioProtoc.1586 浏览次数: 11274
评审: Fanglian HeBenoit Chassaing

相关实验方案
酸水解-高效液相色谱法测定集胞藻PCC 6803中聚3-羟基丁酸酯的含量
Janine Kaewbai-ngam [...] Tanakarn Monshupanee
2023年08月20日 1737 阅读
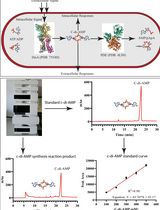
基于高效液相色谱法的史氏分枝杆菌DisA环二腺苷酸(C-di-AMP)合成酶活性研究
Avisek Mahapa [...] Dipankar Chatterji
2024年12月20日 1702 阅读
Abstract
Under aerobic conditions, Staphylococcus aureus (S. aureus) primarily metabolizes glucose to acetic acid. Although normally S. aureus is able to re-utilize acetate as a carbon source following glucose exhaustion, significantly high levels of acetate in the culture media may not only be growth inhibitory but also potentiates cell death in stationary phase cultures by a mechanism dependent on cytoplasmic acidification. One consequence of acetic acid toxicity is the production of reactive oxygen species (ROS). The present protocol describes the detection of ROS in S. aureus undergoing cell death by electron paramagnetic resonance (EPR) spectroscopy. Using 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) as a cell permeable spin probe, we demonstrate the detection of various oxygen radicals generated by bacteria. Although standardized for S. aureus, the methods described here should be easily adapted for other bacterial species. This protocol is adapted from Thomas et al. (2014) and Thomas et al. (2010).
Keywords: EPR (EPR)Materials and Reagents
- Staphylococcus aureus
- Bacto tryptic soy broth without dextrose (TSB) (BD Diagnostic Systems, catalog number: DF0862178 )
- Glucose (Sigma-Aldrich, catalog number: G8270 ).
- 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) (Noxygen Science Transfer & Diagnostics GmbH, catalog number: NOX-2.2-100mg )
- Superoxide dismutase (SOD) (Sigma-Aldrich, catalog number: S7571 )
- Dimethyl thiourea (DMTU) (Sigma-Aldrich, catalog number: D188700 )
- Critoseal (Thermo Fisher Scientific, catalog number: 0267620 )
- 5 µM DETC (Noxygen, Catalog number: NOX-10.1 )
- 25 µM deferoxamine (Noxygen, Catalog number: NOX-10.1)
- Culture flask (250 ml)
- Culture tubes
- 1.5 ml Eppendorf tubes
- Krebs-HEPES buffer (KDD buffer) (see Recipes)
Equipment
- 37 °C shaker-incubator (250 rpm per min)
- Leica Biosystems Critoseal capillary tube sealant (Leica Microsystems, catalog number: MS215003A )
- Bruker e-Scan EPR Spectrometer and Noxygen Temperature Controller Bio-I (Bruker, model: NOX-E.11-ESR )
- Micropipettes (50 µl, EPR tubes) (Noxygen Science Transfer & Diagnostics GmbH, catalog number: MS215003A )
- Spectrophotometer
- Vortex-Genie 2
Software
- Bruker WinEPR Data Processing software
Procedure
文章信息
版权信息
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Thomas, V. C., Chaudhari, S. S., Jones, J., Zimmerman, M. C. and Bayles, K. W. (2015). Electron Paramagnetic Resonance (EPR) Spectroscopy to Detect Reactive Oxygen Species in Staphylococcus aureus. Bio-protocol 5(17): e1586. DOI: 10.21769/BioProtoc.1586.
- Thomas, V. C., Sadykov, M. R., Chaudhari, S. S., Jones, J., Endres, J. L., Widhelm, T. J., Ahn, J. S., Jawa, R. S., Zimmerman, M. C. and Bayles, K. W. (2014). A central role for carbon-overflow pathways in the modulation of bacterial cell death. PLoS Pathog 10(6): e1004205.
分类
微生物学 > 微生物生物化学 > 其它化合物
生物化学 > 其它化合物 > 活性氧
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link