- EN - English
- CN - 中文
Assays of Polyphenol Oxidase Activity in Walnut Leaf Tissue
核桃叶组织中的多酚氧化酶活性分析
发布: 2014年08月20日第4卷第16期 DOI: 10.21769/BioProtoc.1213 浏览次数: 22570
评审: Saminathan ThangasamyZhaohui LiuAnonymous reviewer(s)
Abstract
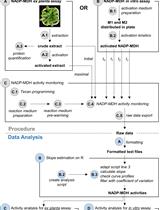
Polyphenol oxidase (PPO) is an enzyme that catalyzes the hydroxylation of monophenols into ortho-diphenols (cresolase activity) and the oxidation of o-diphenols into quinones (catecholase activity) (Figure 1). These quinones spontaneously polymerize to form dark-colored phytomelanins, most often seen in the browning of damaged plant tissue. PPO activity can be easily assayed in crude protein extracts from English walnut (Juglans regia) leaves and from many other plant tissue extracts. PPO activity is most commonly measured by spectrophotometric assay, in which the rate of phytomelanin production is quantified, or by oxygen electrode assay, in which the consumption of oxygen by the enzyme is quantified (Figure 1). Though simpler, the utility of the spectrophotometric assay is limited by variation in the absorption maxima of phytomelanins generated from different phenolic substrates. The oxygen electrode assay is generally considered the “gold standard” for measurement of PPO activity, but it is more time consuming and difficult to implement with monophenol substrates, since cresolase activity is typically quite low compared to catecholase activity. This protocol will describe crude protein extraction from walnut leaves, the spectrophotometric assay, and the oxygen electrode assay for determining PPO activity.
Keywords: Polyphenol oxidase (多酚氧化酶)
Figure 1. The activity of the polyphenol oxidase enzyme
Materials and Reagents
- Protein extraction
- Juglans regia leaf tissue
- Liquid nitrogen
- Insoluble polyvinylpolypyrrolidone (PVPP) (Sigma-Aldrich, catalog number: 25249-54-1 )
- Bovine serum albumin (BSA) (Thermo Fisher Scientific, catalog number: 9048-46-8 )
- Bradford reagent (Bio-Rad Laboratories, catalog number: 500-0006 )
- Tris base (Thermo Fisher Scientific, catalog number: 77-86-1 )
- Citric acid monohydrate (Thermo Fisher Scientific, Acros Organics, catalog number: 5949-29-1 )
- Cysteine hydrochloride (Thermo Fisher Scientific, catalog number: 7048-04-6 )
- Ascorbic acid (Phyto Technology Laboratories®, catalog number: 50-81-7 )
- Polyethylene glycol (PEG) 8000 (Thermo Fisher Scientific, catalog number: 25322-68-3 )
- Glycerol (Thermo Fisher Scientific, catalog number: 56-81-5 )
- Protein extraction buffer (see Recipes)
- Juglans regia leaf tissue
- Spectrophotometric assay
- Catalase (Thermo Fisher Scientific, catalog number: 9001-05-2 )
- Kojic acid (Thermo Fisher Scientific, catalog number: 501-30-4 )
- Monobasic dihydrogen sodium phosphate (Thermo Fisher Scientific, catalog number: 7558-80-7 )
- Dibasic monohydrogen sodium phosphate (Thermo Fisher Scientific, catalog number: 7558-79-4 )
- Sodium dodecyl sulfate (SDS) (Thermo Fisher Scientific, catalog number: 151-21-3 )
- Sodium phosphate buffer containing phenolic substrate (see Recipes)
- Catalase (Thermo Fisher Scientific, catalog number: 9001-05-2 )
- Oxygraph assay
- Kojic acid (Thermo Fisher Scientific, catalog number: 501-30-4)
- Sodium phosphate buffer containing phenolic substrate (see Recipes)
- Assay buffer (see Recipes)
- Kojic acid (Thermo Fisher Scientific, catalog number: 501-30-4)
Equipment
- Ceramic mortar and pestle
- 50 ml centrifuge tubes
- 30 ml Oak Ridge tubes (Thermo Fisher Scientific, Nalgene®, catalog number: 3114-0030 )
- 1.5 ml microfuge tubes
- Spectrophotometer cuvettes or clear, flat bottom 96-well microtiter plates
- Micropipettes
- Vortexer
- Floor centrifuge with rotor that accommodates 30 ml Oak Ridge tubes
- Spectrophotometer or microplate reader (e.g. Molecular Devices SpectraMax M5)
- Oxygen electrode with computer interface (e.g. Hansatech Instruments, Oxygraph)
- -80 °C freezer
Procedure
文章信息
版权信息
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Gertzen, R. and Escobar, M. A. (2014). Assays of Polyphenol Oxidase Activity in Walnut Leaf Tissue. Bio-protocol 4(16): e1213. DOI: 10.21769/BioProtoc.1213.
- Araji, S., Grammer, T. A., Gertzen, R., Anderson, S. D., Mikulic-Petkovsek, M., Veberic, R., Phu, M. L., Solar, A., Leslie, C. A., Dandekar, A. M. and Escobar, M. A. (2014). Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol 164(3): 1191-1203.
分类
植物科学 > 植物生物化学 > 蛋白质 > 活性
植物科学 > 植物新陈代谢 > 酚醛物质
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link