往期刊物2024
卷册: 14, 期号: 16
生物工程
Tetrazine Amino Acid Encoding for Rapid and Complete Protein Bioconjugation
快速且完全的蛋白质生物共轭的四嗪氨基酸编码技术
细胞生物学
Calibrating Fluorescence Microscopy With 3D-Speckler (3D Fluorescence Speckle Analyzer)
使用3D-Speckler校准荧光显微镜(3D荧光散斑分析仪)
发育生物学
Protocol for Imaging the Same Class IV Neurons at Different Stages of Development
在不同发育阶段成像同一类IV型神经元的操作步骤
微生物学
Extraction of Bacterial Membrane Vesicle and Phage Complex by Density Gradient Ultracentrifugation
通过密度梯度超速离心法提取细菌膜泡和噬菌体复合物
分子生物学
Simple Analysis of Gel Images With IOCBIO Gel Software
使用IOCBIO Gel软件简单分析胶图像
神经科学
Using Localization Microscopy to Quantify Calcium Channels at Presynaptic Boutons
使用定位显微技术量化突触前终扣的钙通道
植物科学
In Vitro Hyphal Branching Assay Using Rhizophagus irregularis
利用Rhizophagus irregularis进行体外菌丝分枝实验
系统生物学
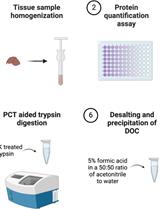
Chloroform/Methanol Protein Extraction and In-solution Trypsin Digestion Protocol for Bottom-up Proteomics Analysis
用于自下而上蛋白质组学分析的氯仿/甲醇蛋白提取与溶液内胰蛋白酶消化方法