往期刊物2023
卷册: 13, 期号: 3
生物化学
Measurement of Secreted Embryonic Alkaline Phosphatase
分泌性胚胎碱性磷酸酶的测定
细胞生物学
Protocol for the Splinted, Human-like Excisional Wound Model in Mice
小鼠仿人切除伤口夹板模型的实验方案
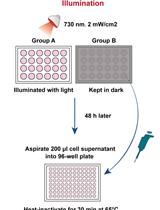
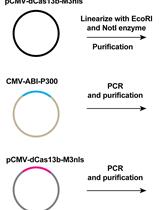
A CRISPR-based Strategy for Temporally Controlled Site-Specific Editing of RNA Modifications
基于 CRISPR 的 RNA 修饰的时间控制位点特异性编辑策略
发育生物学
Dual-Color Live Imaging of Adult Muscle Stem Cells in the Embryonic Tissues of Drosophila melanogaster
果蝇胚胎组织成体肌肉干细胞的双色实时成像
免疫学
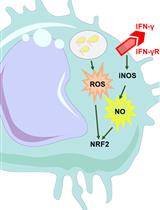
Continuous Measurement of Reactive Oxygen Species Formation in Bacteria-infected Bone Marrow–derived Macrophages Using a Fluorescence Plate Reader
使用荧光酶标仪连续测量受细菌感染的骨髓源性巨噬细胞中活性氧的生成
分子生物学
Isolation of Nuclei from Human Snap-frozen Liver Tissue for Single-nucleus RNA Sequencing
从人速冻肝组织中分离细胞核用于单核 RNA 测序
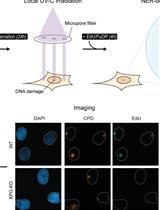
Unscheduled DNA Synthesis at Sites of Local UV-induced DNA Damage to Quantify Global Genome Nucleotide Excision Repair Activity in Human Cells
在局部紫外线诱导的 DNA 损伤位点进行程序外DNA合成,以量化人类细胞中的全基因组核苷酸切除修复活性
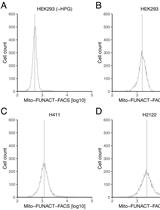
High-throughput Assessment of Mitochondrial Protein Synthesis in Mammalian Cells Using Mito-FUNCAT FACS
使用 Mito-FUNCAT FACS 对哺乳动物细胞中线粒体蛋白合成进行高通量评估
植物科学
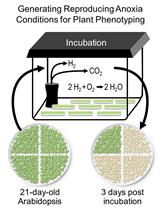
Generating Reproducing Anoxia Conditions for Plant Phenotyping
生成可重复的缺氧条件用于植物表型分析
Assay for Phytaspase-mediated Peptide Precursor Cleavage Using Synthetic Oligopeptide Substrates
使用合成寡肽底物测定植物蛋白酶介导的肽前体裂解