- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Purification of Human Monocytes and Lymphocyte Populations by Counter Current Elutriation – A Short Protocol
Published: Vol 3, Iss 23, Dec 5, 2013 DOI: 10.21769/BioProtoc.981 Views: 12172
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Generation of T cells from Human and Nonhuman Primate Pluripotent Stem Cells
Akhilesh Kumar [...] Igor I. Slukvin
Jul 5, 2020 7286 Views

Expansion and Polarization of Human γδT17 Cells in vitro from Peripheral Blood Mononuclear Cells
Xu Chen [...] Jun Yan
Jan 5, 2024 1951 Views

Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2619 Views
Abstract
Investigations of the activation processes involved in human monocytes and monocyte-derived macrophages and dendritic cells often required large numbers of cells that have not been possibly altered or activated by adherence to surfaces, by binding of antibodies to surface antigens during positive selection, or by release of activators by platelets or other non myeloid cells during isolation or co-culture. Human peripheral blood monocytes as well as lymphocytes from the same blood donor can be isolated by counterflow elutriation using a modification of the technique of Lionetti et al. (1980) as described previously (Bobak et al., 1986). From a unit of blood drawn into anticoagulant, 60-120 million monocytes can be obtained. These cells are not activated and have been shown to be appropriately capable of differential activation in multiple studies.
Keywords: MonocyteMaterials and Reagents
- Peripheral blood from normal human donor (450 ml)
- CPD AS-1 blood collection bags (Citrate-Phosphate-Dextrose, Adenine saline-1) (Baxter)
- 1x PBS-2mM EDTA
- Sterile milliQ H2O
- Lymphocyte separating medium (LSM) (MP Biomedicals, catalog number: 0 850494 )
- HBSS (Fisher Scientific, catalog number: 21023CV )
- Ethanol (70%, 1 liter)
- 10% Bleach (1 liter)
- 10% defined FBS (Hyclone, catalog number: SH30070.03 )
- 1% P/S (Mediatech, CellGro®, catalog number: 30-002-CIRF )
- 1% L-Gln (Mediatech, CellGro®, catalog number: 25-005-CI )
- Recombinant human IL-2 (Pepro Tech, catalog number: 200-02 )
- Recombinant human M-CSF (Pepro Tech, catalog number: 300-25 )
- 25% Albumin (Human), USP (Talecris Plasma Resources, catalog number: 13533-684-20 )
- Complete media (see Recipes)
- 1x sterile PBS (dilute 1:5 from 5x PBS pH 7.0 – 7.2) (see Recipes)
- Ammonium Chloride Potassium (ACK) buffer (see Recipes)
- 1x sterile Elutriation Buffer (see Recipes)
- 5x Phosphate Buffered Saline (see Recipes)
Equipment
- Extruder
- T75 flasks
- Nunc blue cap 50-ml polypropylene tubes (important as monocytes stick to other tubes lowing yields)
- Tubing clamps
- Scissors
- P1000 Pipetteman
- Petri dishes
- 250 ml plasma tube
- Isopropanol wipes, individually packaged
- Beckman JE6B Elutriation System and Rotor (Beckman Coulter, model: JE6B-IM-3) (November 1992)**
- Centrifuge (Sorvall, model: RT6000 or RC-3B )
- Stericup (Fisher Scientific, catalog number: SCGVU11RE )
** Beckman Coulter has newer models Avanti J-301 or J-26S XP with Elutriation Systems, but we have no experience with those.
Procedure
- Receive 450 ml peripheral blood from normal human donor drawn in CPD AS-1
Using an RC-3B Centrifuge, spin a 450 ml bag of blood upright with no brake, 25 °C, 1,665 x g, for 7 min. Takes approximately 30 min to come to a stop without the brake.
- Assemble elutriator and chambers
See Beckman JE6B Elutriation System and Rotor Assembly Manual.
- Sterilize and prepare elutriation chamber
- Attach 1 ml pipet to elutriation pump tubing and transfer tubing (by submerging in each solution) to each of the following in sequence:
- Wash #1 10% fresh bleach (in sterile milliQ H2O)
- Wash #2 300-500 ml: sterile milliQ H2O
- Wash #3 300-500 ml: 70% EtOH (sterile milliQ H2O)
- Spin elutriator rotor by hand for 30 sec to remove air pockets.
- Wash #4 300-500 ml: sterile milliQ H2O
- Wash #5 300-500 ml: sterile PBS
- Wash #1 10% fresh bleach (in sterile milliQ H2O)
- Finally, equilibrate tubing and chamber with elutriation buffer (with 100-200 ml approximately 11-12 ml/min.flow rate).
- Attach 1 ml pipet to elutriation pump tubing and transfer tubing (by submerging in each solution) to each of the following in sequence:
- Collect blood from centrifuge
- Sit blood bag on extruder carefully and place onto the hooks on extruder.
- Wipe tube of blood bag and scissors with alcohol wipes to sterilize. Wipe tubing end with alcohol, attach clamp, and insert into a 250 ml plasma tube (if collecting plasma for later use) or waste (if not collecting plasma).
- With clamp shut on tubing, release press to squeeze bag gently.
- Open clamp to control flow to a fast drip/slow stream. Stop plasma collection when plasma is approximately 1 inch from top of bag.
- Transfer blood bag tube to T75 flask containing 50 ml PBS 0.02 mM EDTA, where you will collect approximately 25 ml plasma and 20 ml erythrocytes plus white cells) often called the buffy coat. Swirl flask gently. Total volume in flask should be approximately 90-100 ml.
- Tilt 50 ml tube containing 14-18 ml LSM and using a 10 ml pipette, layer approximately 30 ml buffy coat-PBS-EDTA carefully from flask onto LSM, repeat with second and third LSM tube.
- Remainder of buffy coat-PBS-EDTA should be layered onto last LSM tube.
- Balance LSM tubes, spin in RT6000 at 350 x g for 40 min, 22 °C with brake off.
- Sit blood bag on extruder carefully and place onto the hooks on extruder.
- Blood/cells
- Remove diluted Plasma from top of tubes of LSM (approximately 25 ml).
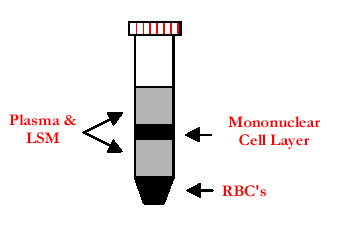
Figure 1. Adapted from http://www.genec.cl/stock/Mediatech.html
- Remove peripheral blood mononuclear cells (PMBC) interfaces, add to 40 ml PBS/1 mM EDTA in 50 ml tubes and invert to mix.
- Spin in RT6000 to remove platelets, 200 x g for 10 min. Pellet should be visible.
- Pour off most of the supernatant.
- Resuspend all pellets, pooling in a total of 8–10 ml elutriation buffer. Avoid air bubbles.
- Count PBMCs: should be between 7 x 108 and 1.5 x 109 total cells depending on the donor.
- Remove diluted Plasma from top of tubes of LSM (approximately 25 ml).
- Elutriation
- Run some elutriation buffer through to equilibrate (100 ml).
- Spin rotor manually for approximately 30 sec to remove air pockets.
- Close lid and allow speed to get up to 1,450 x g (final speed) before checking flow rate: should be between 11.4 and 11.6 ml/min.
- Transfer pipette attached to pump tubing into cells and pump full volume of cells (8-10 ml) before transferring pipette back to elutriation buffer. Avoid taking up any air by pinching tubing during transfer.
- Collect the effluent. Collect the first two 50 ml tubes of effluent which will contain the lymphocyte population (with some contaminating platelets and red cells) and spin down at 100 x g to remove platelets. Lyse any remaining erythrocytes in ACK at room temperature (by resuspending the pellet in the ACK and gently inverting the tube 1-2 times), wash the lymphocytes once in HBSS by resuspending in 25 ml, count cell concentration, pellet cells again at 100 x g, and resuspend the lymphocytes in complete media containing 50 U/ml IL-2 at 2 million/ml in complete media in T75 flasks. Monocytes will remain in chamber, due to size and density and centrifugation conditions.
- After 35 min run at 11.5 ml/min increase speed to 12.5 ml per min. Continue to run for 25 min (total runtime is 60 min).
- Run some elutriation buffer through to equilibrate (100 ml).
- End of run
- Clamp output and input tubes before pump is turned off, then immediately stop centrifuge.
- Open lid and unlock black lock, then remove input tube first, then lower outlet tube.
- Remove elute chamber, with spout up.
- In hood, using a P1000 remove the 6.3 ml of sample from the elutriation chamber and add to 50 ml tube.
- Bring up volume up to 20 ml with 1x PBS. Count cells. Monocytes should be approximately 10-20% of original PBMC count. Wash cell pellet with sterile PBS. 200 x g for 10 min. Resuspend pellet to 100 x 106 cells/ml.
- Plate monocytes in 10 cm petri dishes at 0.5 x 10 E6/ml (10 ml total/dish) in complete media containing 25 ng/ml M-CSF for derivation into macrophages.
- Clamp output and input tubes before pump is turned off, then immediately stop centrifuge.
- Clean elutriator
- Place elute chamber back into rotor and rotor back into centrifuge chamber attaching tubes to wash out/decontaminate rotor, tubing and chamber.
- Undo clamps and run through 300 ml 10% bleach, 300 ml autoclaved H2O, 300 ml 70% EtOH, 300 ml water again. Disassemble rotor, set in 70% ethanol for 30% and let air dry overnight.
- Place elute chamber back into rotor and rotor back into centrifuge chamber attaching tubes to wash out/decontaminate rotor, tubing and chamber.
Recipes
- Complete media
RPMI
10% defined FBS
1% P/S
1% L-Gln
- ACK buffer
To 450 ml milliQ water, add :
4.145 g Ammonium Chloride (annhydrous)
0.5 g potassium bicarbonate
18.6 mg disodium EDTA
Adjust pH to 7.4
Bring final volume to 500 ml with milliQ water
Fliter sterilize (Using Stericup) and store at 4 °C.
- 1x sterile Elutriation Buffer, pH 7.3
8.3 g NaCl
5 g dextrose in 1 L MQ H2O
10 mM Na2HPO4
3 mM NaH2PO4.H2O
100,000 units Pen/Strep
0.625% Human Serum Albumin (HSA) from Talecris Therapeutics
- 5x Phosphate Buffered Saline (PBS for washing cells), pH 7.0-7.2
9.51 g dibasic phosphate, anhydrous (diluted is 6.7 mM Na2HPO4)
4.56 g monobasic phosphate (diluted is 3.3 mM Na H2PO4-H2O)
85 g NaCl (diluted will be .14 M)
Dissolve and bring to final volume of 2000 ml with MilliQ (LPS-free) water. Filter sterilize. Diluted at room temperature should be pH 7.0-7.2, and K = 14-16. Store at 4 °C.
Acknowledgments
The original protocol was published in Bobak et al. (1986). Further modification and development of these methods was supported in part by NIH AI-41090 and T32 AI 60573.
References
- Bobak, D. A., Frank, M. M. and Tenner, A. J. (1986). Characterization of C1q receptor expression on human phagocytic cells: effects of PDBu and fMLP. J Immunol 136(12): 4604-4610.
- Lionetti, F., Hunt, S. and Valeri, C. (1980). Methods of cell separation. Plenum Publishing Corporation, New York.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Clarke, E. V., Benoit, M. E. and Tenner, A. J. (2013). Purification of Human Monocytes and Lymphocyte Populations by Counter Current Elutriation – A Short Protocol. Bio-protocol 3(23): e981. DOI: 10.21769/BioProtoc.981.
Category
Immunology > Immune cell isolation > Lymphocyte
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










