- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessment of Human Dendritic Cell Antigen Uptake by Flow Cytometry
Published: Vol 3, Iss 22, Nov 20, 2013 DOI: 10.21769/BioProtoc.974 Views: 18789
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Epidermal Growth Factor (EGF) Receptor Endocytosis Assay in A549 Cells
Sabrina Rizzolio and Luca Tamagnone
Aug 5, 2013 19326 Views

Detection of Autophagy in Human Peripheral Blood Mononuclear Cells Using Guava® Autophagy and Flow Cytometry
Melanie Scherer [...] Jörg Bergemann
Sep 20, 2025 1417 Views

Protocol for the Isolation and Analysis of Extracellular Vesicles From Peripheral Blood: Red Cell, Endothelial, and Platelet-Derived Extracellular Vesicles
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
Nov 5, 2025 1448 Views
Abstract
Antigen uptake by dendritic cells is the first key step towards induction of antigen-specific T-cell responses. This flow cytometry-based protocol describes the analysis of dendritic cell uptake of soluble antigens through two different mechanisms: non-specific macropinocytosis (using Lucifer Yelloy CH), and receptor-mediated endocytosis (using DQTM Ovalbumin). The protocol is generated based on data presented in Olivar et al. (2013).
Keywords: Dendritic cellsMaterials and Reagents
- Whole blood
- RPMI 1640 Medium, GlutaMAXTM (Gibco®, catalog number: 61870 )
- DPBS without Ca2+ and Mg2+ (Gibco®, catalog number: 14190-169 )
- 100x liquid Penicillin-Streptomycin (Gibco®, catalog number: 15140-122 )
- 200 mM L-Glutamine solution (Gibco®, catalog number: 25030-024 )
- Fetal Bovine Serum (FBS) (Gibco®, catalog number: 10270106 )
- Lipopolysaccharide from Escherichia coli 026:B6 (10 mg) (Sigma-Aldrich, catalog number: L2654 )
- Lucifer Yellow CH dilithium salt (25 mg) (Sigma-Aldrich, catalog number: L0259 )
- DQTM Ovalbumin (1 mg) (Molecular Probes®, catalog number: D-12053 )
- Ficoll-Paque PLUS (General Electric Company, catalog number: 17-1440-03 )
- GMP Recombinant Human Interleukin-4 (50 μg, 13 x 106 IU/mg) (Gentaur Molecular Products, catalog number: 04-GMPhuIL4-50 μg )
- Recombinant Human GM-CSF (300 μg, 3.88 x 106 IU/vial) (Gentaur Molecular Products, catalog number: 04- RHUGM-CSF-300 μg )
- IL-4
- Bovine Serum Albumin Fraction V (BSA) (Roche Diagnostics, catalog number: 10735078001 )
- FITC-conjugated anti-CD14 (RMO52) (Beckman Coulter, catalog number: IM0645U )
- FITC-conjugated anti-IgG2a (7T4-1F5) (Beckman Coulter, catalog number: IM0645U)
- Perfect-Count MicrospheresTM (Cytognos S. L., catalog number: CYT-PCM-50 )
- NaN3 (Sigma-Aldrich, catalog number: 71289 )
- FACS buffer (see Recipes)
- Complete medium (see Recipes)
- DQ-OVA (1 mg/ml) (see Recipes)
- Lucifer Yellow (10 mg/ml) (see Recipes)
- rHuIL-4 (500 IU/ml) (see Recipes)
- rHuGM-CSF (800 IU/ml) (see Recipes)
- LPS (1 mg/ml) (see Recipes)
Equipment
- 15 ml Ficoll-Paque PLUS
- 60-mm cell culture plates (Corning, catalog number: 15 430166 )
- Cytometer tubes (BD Falcon tubes, round-bottom) (Becton, Dickinson and Company, catalog number: 352052 )
- Centrifuge Heraeus Multifuge 3 L-R (Heraeus Holding, catalog number: 75004370 )
- 37 °C, 5% CO2 cell culture incubator
- BD FACSCalibur flow cytometer (Becton, Dickinson and Company, catalog number: 342975 )
Software
- CellQuest Pro software (Becton, Dickinson and Company, catalog number: 643436 )
Procedure
- Dilute 25 ml of buffy coat (initial leukocyte concentrate from a whole blood donation) with the same volume of DPBS.
- Prepare two 50 ml tubes with 15 ml Ficoll-Paque PLUS. Carefully layer 25 ml of the diluted blood sample on Ficoll-Paque PLUS. Important: when layering the sample do not mix Ficoll-Paque PLUS and the diluted blood sample.
- Centrifuge at 400 x g for 25 min at 18-20 °C. Important: brakes off.
- Soak up the white interphase between the diluted plasma fraction and the transparent ficoll fraction with a pipette and transfer it into a fresh tube.
- Wash twice with DPBS.
- Resuspend the pellet in 5 ml DPBS.
- In a cytometer tube mix 3 μl of FITC-conjugated anti-CD14 antibody plus 60 μl DPBS and 20 μl of cellular suspension.
- Incubate 15-18 min at room temperature.
- Add 120 μl DPBS and count the number of CD14+ monocytes by flow cytometry using Perfect-Count MicrospheresTM according to the manufacturer’s instructions.
- Plate monocytes at 1 x 106 cells/ml in 60-mm culture plates, in RPMI 1640 medium without serum, and allow to adhere for 2 h at 37 °C in 5% CO2.
- Remove the non-adherent cells by washing in DPBS. The final population of adherent cells contains 75-80% of monocytes, as demonstrated by flow cytometry of anti-CD14–stained isolates.
- Generate monocyte-derived DCs by supplementing the monocyte cultures with 1 ml of complete RPMI 1640 medium plus GM-CSF (800 IU/ml) and IL-4 (500 IU/ml).
- At day 3 add 1ml of complete RPMI 1640 medium plus GM-CSF (800 IU/ml) and IL-4 (500 IU/ml).
- For DC maturation, at day 5 replace the old medium with fresh complete RPMI 1640 medium plus GM-CSF (800 IU/ml) and IL-4 (500 IU/ml) and stimulate the immature DCs for 48 h with 5 μg/ml LPS.
- Harvest the non-adherent cells floating in the culture medium in a 15 ml tube (at day 5 for immature DCs; at day 7 for mature DCs). Add 2 ml DBPS (37 °C), rinse and collect the adhered cells by pipetting. Wash twice more with DPBS and pool both floating and adherent cells. Centrifugue and resuspend the pellet in 500 μl of complete medium.
- Prepare two cytometer tubes with 60 μl of complete medium plus 4 μl DQ-OVA (stock: 1 mg/ml) at 37 °C or 0 °C.
- Prepare two cytometer tubes with 60 μl of complete medium plus 6 μl Lucifer Yellow CH (stock: 10 mg/ml) at 37 °C or 0 °C.
- Add 100 μl of cell suspension (~ 2 x 105 cells/ml) to each cytometer tube.
- Incubation time: 15 min for DQ-OVA; 120 min for Lucifer Yellow CH. The fluorescence of OVA labeled with BODIPY FL dye (DQ-OVA) is self-quenched until the OVA is taken up via the mannose receptor and degraded only by endolysosomal proteases. Lucifer Yellow CH (LY) is a hydrophilic tracer for fluid-phase macropinocytosis. LY is not degraded and is nontoxic at concentrations up to 6 mg/ml.
- Stop the incubations by adding 1 ml cold FACS buffer.
- Wash the cells two times with cold FACS buffer.
- Analyze the incorporated fluorescence of both immature DCs (Figure 1) and mature DCs using flow cytometry. Compare the histograms and corresponding mean fluorescence intensities (MFI) between cells incubated at 37 °C (specific uptake) and cells incubated at 0 °C (non-specific uptake: passive diffusion,…).
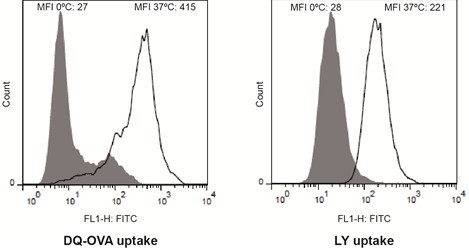
Figure 1. Analysis of the endocytic activity of immature DCs by flow cytometry. The endocytic activity of monocyte-derived immature DCs was assessed measuring the uptake of the fluorescent reporters DQ-OVA (receptor-mediated endocytosis) and Lucifer Yellow CH (fluid-phase endocytosis). Representative histograms are shown. Dye uptake controls are displayed in gray. The median fluorescence intensities (MFI) for the different fluorescent cell populations are indicated in each histogram.
Recipes
- FACS buffer (500 ml)
Mix 5 g BSA and 0.5 g NaN3 with 500 ml 1x DPBS
Filter sterilize (0.45 μm)
Stored at 4 °C
- Complete medium
RPMI 1640 medium, GlutaMAXTM
100 μg/ml streptomycin
100 IU/ml penicillin
2 mM L-glutamine
10% heat-inactivated FBS
GM-CSF 800 IU/ml
IL-4 500 IU/ml
Stored at 4 °C
- DQ-OVA (1 mg/ml)
A 1 mg/ml solution can be prepared by dissolving the contents of one vial in 1 ml of DPBS. Once reconstituted, the solution should be stored at -20 °C, protected from light.
- Lucifer Yellow (10 mg/ml)
A 10 mg/ml solution can be prepared by dissolving the contents of one vial in 2.5 ml of dH2O. Once reconstituted, the solution should be stored at 4 °C, protected from light.
- rHuIL-4 (500 IU/ml)
A 500 IU/ml solution can be prepared by dissolving the contents of one vial in 500 μl of dH2O. Once reconstituted, the solution should be stored at -80 °C.
- rHuGM-CSF (800 IU/ml)
A 800 IU/ml solution can be prepared by dissolving the contents of one vial in 2 ml of dH2O. Once reconstituted, the solution should be stored at -80 °C.
- LPS (1 mg/ml)
A 1 mg/ml solution can be prepared by dissolving the contents of one vial in 1 ml of DPBS. Once reconstituted, the solution should be stored at -20 °C.
Acknowledgments
This protocol was adapted from the previously published study, Olivar et al. (2013), and was supported by the Ministerio de Ciencia e Innovación (Madrid, Spain), through grant PI10/1073 from the “Fondo de Investigaciones Sanitarias” (FIS-ISCIII), and from 2009SGR1490 (Generalitat de Catalunya) to JMA. JMA is sponsored by the “Researchers Consolidation Program” from the SNS-Dpt. Salut Generalitat de Catalunya (Exp. CES06/012).
References
- Olivar, R., Luque, A., Naranjo-Gomez, M., Quer, J., Garcia de Frutos, P., Borras, F. E., Rodriguez de Cordoba, S., Blom, A. M. and Aran, J. M. (2013). The α7β0 isoform of the complement regulator C4b-binding protein induces a semimature, anti-inflammatory state in dendritic cells. J Immunol 190(6): 2857-2872.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Luque, A., Cárdenas-Brito, S., Olivar, R. and Aran, J. M. (2013). Assessment of Human Dendritic Cell Antigen Uptake by Flow Cytometry. Bio-protocol 3(22): e974. DOI: 10.21769/BioProtoc.974.
Category
Immunology > Immune cell function > Dendritic cell
Cell Biology > Cell-based analysis > Flow cytometry
Cell Biology > Cell-based analysis > Cytosis > Endocytocis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









