- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cell Fractionation and Quantitative Analysis of HIV-1 Reverse Transcription in Target Cells
Published: Vol 3, Iss 20, Oct 20, 2013 DOI: 10.21769/BioProtoc.949 Views: 10954
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A SYBR Green-based Real Time RT-PCR Assay for Detection of the Emerging H7N9 Virus
Zheng Zhu and Lunbiao Cui
Jun 20, 2014 10104 Views

Infectious Virus Yield Assay for Hepatitis E Virus
Yannick Debing [...] Johan Neyts
Aug 5, 2014 11477 Views

Quantification of HIV RNA and Human Herpesvirus DNA in Seminal Plasma
Milenka V. Vargas-Meneses [...] Sara Gianella
May 5, 2015 11130 Views
Abstract
This is a protocol to detect HIV-1 reverse transcription products in cytoplasmic and nuclear fractions of cells infected with VSV-G-pseudotyped envelope-defective HIV-1. This protocol can also be extended to HIV-1 with regular envelope.
Materials and Reagents
- HEK 293T cells
- HeLa cells
- Dulbecco’s Modified Eagle Medium (DMEM) (Mediatech, Cellgro®, catalog number: 10-013-CV )
- R9-ΔE plasmid ((Zhou and Aiken, 2001), an HIV-1 proviral DNA clone created by introducing a frameshift mutation in envelope of the wild-type infectious R9 clone. Virions produced by this clone are non-infectious but can be made infectious by pseudotyping with envelopes from VSV or other viruses)
- pHCMV-G (VSV-G) plasmid ((Yee et al., 1994), a retrovirus-derived plasmid in which the retroviral envelope glycoprotein is replaced with glycoprotein from vesicular stomatitis virus [VSV]).
- p24 ELISA kit (in-house)
- Phosphate-buffered saline (PBS) (Mediatech, Cellgro®, catalog number: 21-0310-CV )
- VSV-G-pseudotyped envelope-defective HIV-1 (R9-ΔE) virus particles
- Efavirenz (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID,
NIH, catalog number: 11680 )
- DNase I (Roche, catalog number: 10104159001 )
- 0.25% Trypsin/2.21 mM EDTA (Mediatech, Cellgro®, catalog number: 25-053-CI )
- Triton X-100 (Mallinckrodt, catalog number: 9002-93-1 )
- DNeasy blood and tissue kit (QIAGEN, catalog number: 69506 )
- cOmplete, Mini, EDTA-free protease-inhibitor cocktail tablet (Roche, catalog number: 11836170001 )
- 4 to 20% Polyacrylamide gradient Tris-glycine gels (Bio-Rad)
- Nitrocellulose membrane (General Electric Company)
- Mouse monoclonal anti-GAPDH antibody (Santa Cruz, catalog number: sc-47724 )
- Mouse monoclonal anti-LaminB1 antibody (Life Technologies, catalog number: 33-2000 )
- SYBR green (ABI, catalog number : 4309155 )
- DpnI (New England Biolabs, catalog number: R0176L )
- DTT
- Yeast tRNA (Roche, catalog number : 10109541001 )
- Forward primer MH531 (5’-TGTGTGCCCGTCTGTTGTGT-3’)
- Reverse primer MH532 (5’-GAGTCCTGCGTCGAGAGAGC-3’)
- DNase/RNase-free water
- SDS-PAGE sample buffer
- Sodium deoxycholate (Sigma-Aldrich, catalog number: 30970 )
- N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid (BES) (Sigma-Aldrich, catalog number: B4554 )
- Hypotonic buffer (see Recipes)
- Radioimmunoprecipitation buffer (see Recipes)
- 2x BES-buffered saline (BBS) (see Recipes)
Equipment
- 10 cm cell culture dish
- 0.45-μm-pore-size syringe filters (Thermo Fisher Scientific, catalog number: 190-2545 )
- 0.20- μm-pore-size syringe filters (Thermo Fisher Scientific, catalog number: 190-2520 )
- 1.5 ml screw-cap tube
- Tabletop centrifuge (Thermo Fisher Scientific, Sorvall®)
- Tabletop refrigerated centrifuge (Thermo Fisher Scientific)
- Mx-3000p thermocycler (Stratagene)
- CO2 incubator
Procedure
- Production of VSV-G-pseudotyped envelope-defective HIV-1 (R9-ΔE clone) virus particles (Aiken, 1998)
- Culture 293T cells in DMEM containing 10% v/v fetal bovine serum (FBS) and supplemented with antibiotics [Penicillin (100 IU/ml) and Streptomycin (100 μg/ml)] at 37 °C, 5% CO2.
- Detach cells from a nearly confluent culture dish with the help of 0.25% Trypsin/2.21 mM EDTA and seed 2 x 106 cells in 9 ml medium per 100 mm culture dish and incubate at 37 °C.
- Transfect of 293T cells next day using the calcium phosphate-BBS method (Chen and Okoyama, 1987).
- Mix 15 μg of R9-ΔE and 5 μg of pHCMV-G (VSV-G) plasmids in a tube.
- Add 0.2 μm filtered water to the tube to make up the volume to 450 μl.
- Add 50 μl of 2.5 M CaCl2 to the tube.
- Add 500 μl of 2x BBS to the tube dropwise.
- Gently mix the contents of the tube by pipetting few times.
- Incubate the tube at room temperature for 20 to 30 min.
- Add the mixture to 293T cells with gentle swirling and incubate cells at 35 °C and 3% CO2.
- Mix 15 μg of R9-ΔE and 5 μg of pHCMV-G (VSV-G) plasmids in a tube.
- Aspirate media from the transfected dish ~16 h after transfection, wash cells with 5 ml PBS, replenish with 5 ml of fresh cell culture media and incubate at 37 °C, 5% CO2.
- Two days after transfection, harvest culture supernatant containing virus particles, centrifuge at 1,500 x g for 5 min to pellet cells and debris.
- Filter the supernatant through 0.45-μm-pore-size syringe filters, aliquot and freeze at -80 °C.
- Culture 293T cells in DMEM containing 10% v/v fetal bovine serum (FBS) and supplemented with antibiotics [Penicillin (100 IU/ml) and Streptomycin (100 μg/ml)] at 37 °C, 5% CO2.
- Infection of HeLa cells with VSV-G-pseudotyped envelope-defective HIV-1 (R9-ΔE)
- Plate HeLa cells at a density of 1.5 x 105 cells/well in 12-well plates (1 ml total culture volume per well).
- 24 hours later treat virus inocula with DNase I (20 μg/ml) plus MgCl2 (10 mM) and incubate in a water bath at 37 °C for 1 h.
- Infect cells with DNase I-treated inocula equivalent to 15 ng of p24 (determined by p24 ELISA using in-house kit (Wehrly and Chesebro, 1997)).
- Perform parallel infection in the presence of efavirenz (1 μM) to define the residual plasmid DNA levels carried over from transfection.
- Incubate infected cells at 37 °C for 8 h.
Note: One can also analyse time course of reverse transcription by harvesting infected cells at different time intervals after infection.
- Plate HeLa cells at a density of 1.5 x 105 cells/well in 12-well plates (1 ml total culture volume per well).
- Cell fractionation of HIV-1 infected HeLa cells
- After incubation for desired time, aspirate culture media and wash cells once with PBS.
- Dislodge adherent cells by incubation with 500 μl of 0.25% Trypsin-EDTA at 37 °C for 2 min.
- Collect trypsinized cells in a 1.5 ml screw-cap tube. Centrifuge at 300 x g for 5 min to pellet cells.
- Lyse cell pellets in 200 μl of hypotonic buffer containing 0.1% Triton-X-100 and incubate on ice for 15 min.
Note: Concentration of Triton-X-100 was optimized for HeLa cells. The concentration of Trition X-100 represents the lowest concentration at which about 95% of the cells counted under the microscope had intact nuclei but no plasma membrane.
- Centrifuge at 17,000 x g for 5 min at 4 °C and collect the supernatant as cytoplasmic fraction.
- Wash the nuclear pellet with 1 ml hypotonic buffer without Triton-X-100 thrice. After each wash centrifuge at 17,000 x g for 5 min at 4 °C to pellet the nuclei and aspirate off supernatant.
- Isolate DNA from nuclear pellet using DNeasy blood and tissue kit as per manufacturer’s protocol. Elute DNA in the last step in a fresh collection tube using 100 μl DNase/RNase-free water. Eluted DNA can be stored at -80 °C or used directly to perform qPCR.
- In parallel, prepare whole-cell, cytoplasmic and nuclear lysates from uninfected cells to check for cytoplasmic contamination of nuclear fractions.
- To prepare whole cell lysate, lyse cells in radioimmunoprecipitation (RIPA) buffer (follow steps C2-C5 except the use of RIPA buffer instead of hypotonic buffer). Add equal volume of 2x SDS-PAGE sample buffer for gel electrophoresis and heat at 95 °C in a heat block for 5 min.
- Prepare cytoplasmic lysate as described above (steps C2 to C5). Add equal volume of 2x SDS-PAGE sample buffer for gel electrophoresis and heat at 95 °C in a heat block for 5 min.
- To prepare nuclear lysate, follow steps C2 to C6, and then lyse the nuclear pellet in 1x SDS-PAGE sample buffer. Heat at 95 °C in a heat block for 5 min. Resolve equal volumes of whole cell, cytoplasmic and nuclear lysates on a 4-20% polyacrylamide gradient Tris-glycine gel.
- Transfer resolved proteins onto a nitrocellulose membrane.
- Block the membrane with 5% non-fat milk solution in PBS and probe with anti-GAPDH and anti-LaminB1 antibodies (concentrations recommended by manufacturer) followed by appropriate secondary antibodies (concentrations recommended by manufacturer) as cytoplasmic and nuclear markers respectively.
- After incubation for desired time, aspirate culture media and wash cells once with PBS.
- SYBR green-based Quantitative PCR for quantitation of viral reverse transcription products
- Treat isolated DNA from step C7 with DpnI (17 μl DNA + 2 μl buffer + 1 μl of DpnI-20 units) by incubation at 37 °C for 1 to 2 h. Inactivate DpnI by incubation at 80 °C for 20 min.
- Quantitation of viral reverse transcription products.
- Prepare reaction mixture by mixing DNA (5 μl), PCR mix containing SYBR green (12.5 μl), forward primer (150 nM), reverse primer (150 nM) and tRNA (1 μg/μl) containing DNase/RNase-free water up to 25 μl.
- Prepare standards ranging from 10 to 109 copies/reaction of R9-ΔE plasmid. Dilutions of standards should be made in 1 μg/μl tRNA-containing water.
- Set PCR reaction using the following thermal profile:
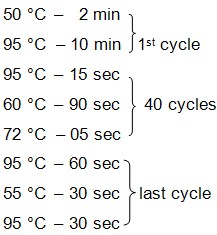
- Prepare reaction mixture by mixing DNA (5 μl), PCR mix containing SYBR green (12.5 μl), forward primer (150 nM), reverse primer (150 nM) and tRNA (1 μg/μl) containing DNase/RNase-free water up to 25 μl.
- Treat isolated DNA from step C7 with DpnI (17 μl DNA + 2 μl buffer + 1 μl of DpnI-20 units) by incubation at 37 °C for 1 to 2 h. Inactivate DpnI by incubation at 80 °C for 20 min.
Recipes
- Hypotonic buffer
10 mM Tris pH 8.0
10 mM KCl
1.5 mM MgCl2
1 mM DTT
Protease inhibitor cocktail (one tablet per 10 ml of buffer)
- Radioimmunoprecipitation buffer
50 mM Tris pH 7.5
1% Triton-X-100
250 mM NaCl
5 mM EDTA
0.1% SDS
1% sodium deoxycholate
Protease inhibitors cocktail (one tablet per 10 ml of buffer)
- 2x BES-buffered saline (BBS)
50 mM BES (N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid)
1.5 mM Na2HPO4
280 mM NaCl
pH 6.95
Acknowledgments
This protocol is adapted from Shah et al (2013). This protocol was supported by NIH grant AI076121 to C.A.
References
- Aiken, C. (1998). Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity. Virology 248(1): 139-147.
- Chen, C. and Okayama, H. (1987). High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7(8): 2745-2752.
- Shah, V. B., Shi, J., Hout, D. R., Oztop, I., Krishnan, L., Ahn, J., Shotwell, M. S., Engelman, A. and Aiken, C. (2013). The host proteins transportin SR2/TNPO3 and cyclophilin A exert opposing effects on HIV-1 uncoating. J Virol 87(1): 422-432.
- Wehrly, K. and Chesebro, B. (1997). p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods 12(4): 288-293.
- Yee, J. K., Friedmann, T. and Burns, J. C. (1994). Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol 43 Pt A: 99-112.
- Zhou, J. and Aiken, C. (2001). Nef enhances human immunodeficiency virus type 1 infectivity resulting from intervirion fusion: evidence supporting a role for Nef at the virion envelope. J Virol 75(13): 5851-5859.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Shah, V. B. and Aiken, C. (2013). Cell Fractionation and Quantitative Analysis of HIV-1 Reverse Transcription in Target Cells. Bio-protocol 3(20): e949. DOI: 10.21769/BioProtoc.949.
Category
Microbiology > Microbial genetics > RNA > qRT-PCR
Microbiology > Microbe-host interactions > Virus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










