- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Protocol for T-cell Adhesion Strength on Tumor Cells under Flow Conditions
Published: Vol 3, Iss 20, Oct 20, 2013 DOI: 10.21769/BioProtoc.936 Views: 10601
Reviewed by: Lin FangFanglian HeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Transient Transfection-based Cell Adhesion Assay with 293T Cells
Rohit Singh and Beom K. Choi
Jan 5, 2021 7357 Views

Carboxyfluorescein Dye Uptake to Measure Connexin-mediated Hemichannel Activity in Cultured Cells
Joe A. Potter [...] Paul E. Squires
Feb 5, 2021 5325 Views

Improved Immunohistochemistry of Mouse Eye Sections Using Davidson's Fixative and Melanin Bleaching
Anne Nathalie Longakit [...] Catherine D. Van Raamsdonk
Nov 20, 2025 1573 Views
Abstract
This method allows evaluating the relative adhesion strength between T lymphocytes and specific adherent target cells using a shear force in flow chambers. It is based on the measure of the resistance of conjugates formed between T cells and adherent tumor cells to shear stress in a microfluidic system. For this purpose, T cells, stained with a CellTracker probe, are added into flow channels containing a monolayer of adherent target cells and their progressive detachment under a constant shear stress is then recorded using a fluorescent microscope.
Materials and Reagents
- Adherent tumor cells [such as non-small cell lung carcinoma (NSCLC) cell lines]
- Specific T-cell clones (generated either from autologous tumor-infiltrating T lymphocytes (TIL) or peripheral blood lymphocytes (PBL))
- RPMI 1640 (Life Technologies, Gibco®, catalog number: 61870044 )
- DMEM-F12 (Life Technologies, Gibco®, catalog number: 31331093 )
- UltroserG (Pall, catalog number: 15950-017 )
- Fetal Bovin Serum (Life Technologies, Gibco®, catalog number: 10270-106 )
- Human serum AB (Institut de Biotechnologies Jacques Boy)
- Penicillin and streptomycin (Life Technologies, Gibco®, catalog number: 15140122 )
- Sodium Pyruvate (Life Technologies, Gibco®, catalog number 11360029 )
- IL-2
- 10x PBS (Life Technologies, Gibco®, catalog number: 70011-036 )
- CellTracker probe (CellTrackerTM Green CMFDA) (Life Technologies, Invitrogen™, catalog number: C2925 )
- Complete DMEM: tumor cell culture medium (LC medium) (see Recipes)
- RPMI-based T-cell complete medium (see Recipes)
Equipment
- Microscope Zeiss LSM-510 (ZEISS) with a heated incubation chamber and CO2 supply
- Micro-Slides VI, ibiTreat (ibidi GmbH, catalog number: 80606 ), two silicon tubes (1.6 mm of inner diameter) with a plastic clip, two Elbow Luer connectors (ibidi GmbH, catalog number: 80646 )
- Syringe pump (high flow rate > 50 ml/min)
- 60 ml syringe (Becton, Dickinson and Company, catalog number: 300866 )
- Humidified incubator at 37 °C with 5% CO2
- A recipient for waste flow buffer (Erlenmeyer)
- Centrifuge (Beckman Coulter, model: GS-6R )
Procedure
- Adherent tumor cell preparation
- Seed adherent tumor cells into IBIDI channels by adding 60 μl of tumor cell suspension in LC medium. Micro-slides VI, ibiTreat characteristics are the following:
Number of channels: 6
Minimal volume per channel: 30 μl
Channel length: 17 mm
Channel width: 3.8 mm
Channel height: 0.4 mm
Growth area: 0.6 cm2 per channel
Tumor cell concentration may vary according to the cell type (for instance: 1.6 x 106 cells/ml for NSCLC cell lines described in Reference 1). Cells should be at 90-95% of confluence the day of experiment. - Incubate the IBIDI slide in a humidified incubator at 37 °C for at least 2 h for cell attachment.
- Fill gently the reservoirs with another 60 μl of LC medium. Avoid pipetting directly into the channels not to detach the cells.
- Incubate overnight at 37 °C, 5% CO2.
Note: In case of tumor cell treatment (example siRNA transfection), cells should be plated two days before using the same experimental conditions. Medium may need to be changed every 24 h. Be sure that the cells are all alive and just reaching 90-95% confluence the day of experiment.
- Seed adherent tumor cells into IBIDI channels by adding 60 μl of tumor cell suspension in LC medium. Micro-slides VI, ibiTreat characteristics are the following:
- T-cell preparation
- Wash T cells with PBS 1x by centrifugation at 350 x g for 5 min.
- Stain cells with CellTracker Green (CMFDA) according to the manufacturer’s protocol. Briefly, resuspend cells at 2 x 106/ml in PBS and add one volume of CellTracker Green (CMFDA 2x) diluted in PBS (final concentration 1 μM). Incubate for 15 min at 37 °C.
- Wash T cells twice with RPMI-based complete medium by centrifugation at 350 x g, 5 min.
- Resuspend T cells in T-cell medium, at final concentration 2 x 106 cells/ml, in 24 flat bottom well plates.
- Incubate T cells overnight in humidified incubator at 37 °C with 5% CO2.
- Wash T cells with PBS 1x by centrifugation at 350 x g for 5 min.
- T-cell adhesion strength under flow conditions
- The following day, warm the thermostatic chamber of the microscope at 37 °C and 5% CO2 (Figure 1A).
- Equilibrate RPMI-based complete medium (500 ml) inside the incubator at 37 °C and 5% CO2.
- Prepare syringe pump (Figure 1B).
- Connect the tube carrying a plastic clip (position closed) to the syringe and fill the syringe with prewarmed medium. Put the Elbow Luer connector and prime the tube (Figure 1A).
- Put the IBIDI slide (Figure 1C) under the microscope objective (20x) (Figure 1D). Be sure that the reservoirs are completely full. If not, add some medium.
- Connect the tube to one extremity of the IBIDI channel making sure there are no air bubbles remaining inside. This step is critical, because bubbles increase the risk of tumor cell detachment, influence the flow rate and can even stop the flow.
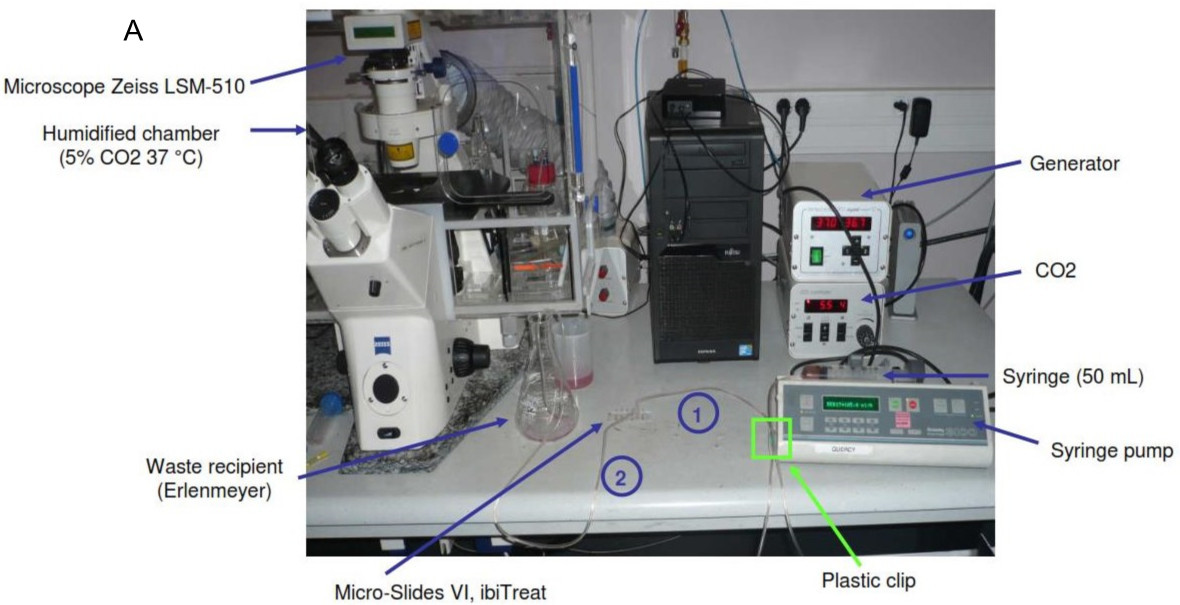
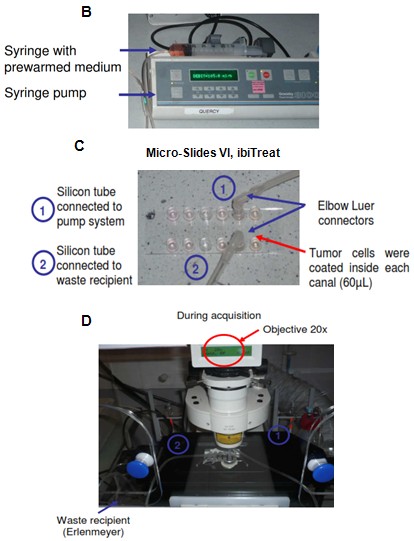
Figure 1. Flow system. A. Whole flow system; B. Pump system; C. Details of Micro-Slides IBIDI connections; D. During acquisition, the slide is fixed under the microscope and connected to the pump system. - Use the second tube to connect the opposite extremity of the channel with a bottle collecting wastes (Figure 2).
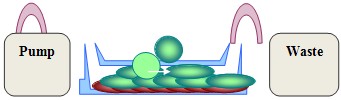
Red: adherent tumor cell layer
Green: T cells
Blue: LC medium
White arrow: direction of the flow
Figure 2. T-cell adhesion under flow conditions. Stained T lymphocytes were incubated for 15 min on a monolayer of autologous tumor cells previously seeded into IBIDI channels. The IBIDI slide is then connected by silicon tubes, in one side to a pump (with a syringe filled with prewarmed medium) and in the other side to the waste recipient. - Release the clip on the tube connecting the syringe to the IBIDI slide (Figure 1A).
Note: Test the system to validate the maximal flow rate that doesn’t detach tumor cells. This rate will be applied to determine the T-cell adhesion strength on tumor cells. - Wash T cells with RPMI by centrifugation at 350 x g for 5 min.
- Resuspend T cells in RPMI medium at final concentration 2 x 106 cells/ml.
- Replace the medium filling the channels with 50 μl of T cells suspension. Be careful to not detach tumor cells or introduce bubbles inside channels.
- Incubate 15 min at 37 °C.
- After incubation, prepare the flow system using the same conditions described for the test assay.
- Add 50 ml pre-warmed medium inside the syringe.
- Start the acquisition just before the flow (Figure 1D).
- Acquire images every 2 s for 60 s. It is expected that T cells adhere more firmly to tumor cells that express adherence molecules (such as ligands for integrins expressed by T cells) than tumor cells that do not express these molecules. (Figure 3)
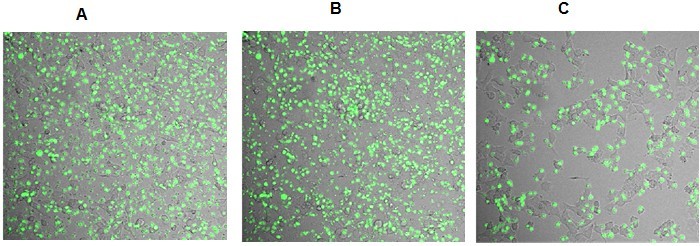
Figure 3. Representative images acquired at different time lapses during T cell adhesion protocol. A. 0 sec; B. 250 sec; C. 640 sec at the flow rate of 100 ml/h.
- The following day, warm the thermostatic chamber of the microscope at 37 °C and 5% CO2 (Figure 1A).
Recipes
- Complete DMEM: tumor cell culture medium (LC)
DMEM-F12 supplemented with:
10% decomplemented Fetal Bovine Serum
1% UltroserG
1% Penicillin and streptomycin
1% Sodium pyruvate - RPMI-based T-cell complete medium
RPMI 1640 complemented with 10% Human serum AB
1% Penicillin and streptomycin
1% Sodium pyruvate
IL-2 (100 U/ml)
Acknowledgments
We thank Sophie Salomé-Desmoulez for her help with confocal microscopy. This work was supported by grants from the INSERM, the Association pour la Recherche sur le Cancer (ARC), the Institut National du Cancer (INCa), the Ligue contre le Cancer and the Cancéropôle Ile de France (IDF). MB is a recipient of a fellowship from the Cancéropôle IDF.
References
- Bernard, G., Raimondi, V., Alberti, I., Pourtein, M., Widjenes, J., Ticchioni, M. and Bernard, A. (2000). CD99 (E2) up-regulates α4β1-dependent T cell adhesion to inflamed vascular endothelium under flow conditions. Eur J Immunol 30(10): 3061-3065.
- Franciszkiewicz, K., Le Floc'h, A., Boutet, M., Vergnon, I., Schmitt, A. and Mami-Chouaib, F. (2013). CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res 73(2): 617-628.
- Rosenthal-Allieri, M. A., Ticchioni, M., Breittmayer, J. P., Shimizu, Y. and Bernard, A. (2005). Influence of β1 integrin intracytoplasmic domains in the regulation of VLA-4-mediated adhesion of human T cells to VCAM-1 under flow conditions. J Immunol 175(2): 1214-1223.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Boutet, M., Franciszkiewicz, K., Floc’h, A. L. and Mami-Chouaib, F. (2013). Protocol for T-cell Adhesion Strength on Tumor Cells under Flow Conditions. Bio-protocol 3(20): e936. DOI: 10.21769/BioProtoc.936.
Category
Cancer Biology > General technique > Cell biology assays > Cell adhesion
Cell Biology > Cell imaging > Fluorescence
Immunology > Immune cell function > Lymphocyte
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











