- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Stomatal Bioassay in Arabidopsis Leaves
Published: Vol 3, Iss 19, Oct 5, 2013 DOI: 10.21769/BioProtoc.921 Views: 16618
Reviewed by: Tie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualization of Actin Organization and Quantification in Fixed Arabidopsis Pollen Grains and Tubes
Xiaolu Qu [...] Shanjin Huang
Jan 5, 2020 5356 Views

Targeting Ultrastructural Events at the Graft Interface of Arabidopsis thaliana by A Correlative Light Electron Microscopy Approach
Clément Chambaud [...] Lysiane Brocard
Jan 20, 2023 2981 Views

Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
Yuta Kato [...] Mitsutaka Taniguchi
Aug 5, 2025 2323 Views
Abstract
Stomata embedded in the epidermis of terrestrial plants are important for CO2 absorption and water transpiration, and are possible points of entry for pathogens. Thus, the regulation of stomatal apertures is extremely important for the survival of plants. Furthermore, stomata can respond via accurate change of stomatal apertures to a series of extracellular stimuli such as phytohormones, pathogens, ozone, drought, humidity, darkness, CO2, visible light and UV-B radiation, so stomatal bioassay is widely used to dissect signal transduction mechanisms of plant cells in responses to multiple stimuli. This protocol describes how to measure stomatal apertures in leaves of model plant Arabidopsis thaliana under multiple treatments.
Materials and Reagents
- Leaves of Arabidopsis (Arabidopsis thaliana)
- Ethanesulfonic acid (Mes)-KOH
- MES/KCl buffer, pH 6.15 (see Recipes)
Equipment
- Light microscope
- Eyepiece micrometer and stage micrometer
- Glass slide and cover glass
- 6-cm diameter Petri plates
- Eyelbrow brush (Yellow wolf hair, length of hair: 0.8 cm, width of hair: 0.8 cm)
- Tweezers
Procedure
- Sampling
Arabidopsis seedlings were gown in plant growth chambers under a 16-h light/8-h dark cycle, a photon flux density of 0.1 mmol/m2/s, and a day/night temperature cycle of 18 °C/22 °C for 4-6 weeks. The youngest, fully expanded and flat leaves were harvested for immediate use.
Notes:- To avoid any potential rhythmic effects on stomatal aperture, sampling was always started at the same time of day and avoided at midday.
- For all treatments, the leaves at same developmental stage were always sampled.
- For each treatment, at least three leaves originated from different plants and at same developmental stage were harvested.
- To avoid any potential rhythmic effects on stomatal aperture, sampling was always started at the same time of day and avoided at midday.
- Opening the stomata
For stomatal closing experiments, to ensure stomata at fully opened stage before starting of treatments, the fresh sampled flat leaves were first floated with their abaxial surfaces facing up on MES/KCl buffer (15 ml) in 6-cm diameter Petri plates for 2-3 h at 22 °C under light condition (0.1 mmol/m2/s) to open the stomata, and then for subsequent treatments. - Treating samples
Once the stomata were fully open (checked by microscope as the following 5 procedure), the leaves were then floated on MES/KCl buffer alone or containing various compounds or inhibitors for required time at 22 °C under the same white light condition mentioned above or under the desired conditions. Control treatments involved addition of buffer or appropriate solvents used with inhibitors.
Notes:- For each treatment, at least three leaves originated from different plants were treated.
- As the epidermal strips is easier peeled from the abaxial surface than the paraxial surface of Arabidopsis leaves, we only peeled epidermal strips from abaxial surface of leaves for subsequent measurement of stomatal aperture. Thus, for treatments of UV-B radiation as well as other lights, to ensure the abaxial surface of leaves receiving same dose of UV-B radiation as well as other lights, the leaves were floated with their abaxial surfaces facing up and perpendicular to the light on MES/KCl buffer in all treatments including opening stomata.
- For each treatment, at least three leaves originated from different plants were treated.
- Peeling epidermal strips and making slides
After the above treatments, the leaf was taken out from MES/KCl buffer and a piece of filter paper was used to absorb the MES/KCl buffer on the surface of leaf. The leaf were flatly placed on a glass slide with its abaxial surfaces facing up, a tweezers was used to clamp a part of abaxial epidermis and mesophyll cells near the tip of leaf and the epidermal strips were quickly peeled along with the direction of the main leaf veins. Then, the peeled epidermal strips were immediately immersed in the corresponding treated buffer and pushed on the bottom of Petri plates by a forceps, the remained mesophyll cells were gently removed from epidermal strips by an eyebrow brush (Figure 1), and the tip of epidermal strip clamped by tweezers with more mesophyll cells was cut off, then slides were made with the corresponding treating buffer.
Figure 1. Eyebrow brush - Measurement of stomatal apertures with a light microscope
Install eyepiece micrometer and stage micrometer in your used light microscope, calibrate micrometer scale, and fifty to ninety randomly selected stomatal apertures were scored under the calibrated microscope in each replicate and treatments were repeated at least three times. The data are presented as means ± SE derived from one-way ANOVA.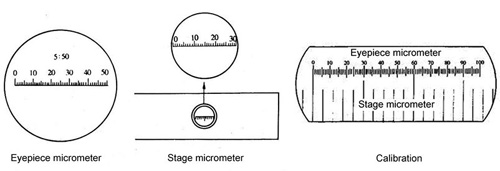
Figure 2. Eyepiece micrometer and stage micrometer
Recipes
- MES/KCl buffer, pH 6.15 (500 ml)
1.86375 g 50 mM KCl
5.549 mg 0.1 mM CaCl2
1.066 g 10 mM Mes-KOH (pH 6.15)
Stored at room temperature and used for one week
Acknowledgments
This work was supported by the National Science Foundation of China (grant no. 31170370) and the Fundamental Research Funds for the Central Universities (grant no. GK200901013). This protocol was adapted from previously published paper He et al. (2013).
References
- He, J. M., Ma, X. G., Zhang, Y., Sun, T. F., Xu, F. F., Chen, Y. P., Liu, X. and Yue, M. (2013). Role and interrelationship of Galpha protein, hydrogen peroxide, and nitric oxide in ultraviolet B-induced stomatal closure in Arabidopsis leaves. Plant Physiol 161(3): 1570-1583.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Li, X., Ma, X. and He, J. (2013). Stomatal Bioassay in Arabidopsis Leaves. Bio-protocol 3(19): e921. DOI: 10.21769/BioProtoc.921.
- He, J. M., Ma, X. G., Zhang, Y., Sun, T. F., Xu, F. F., Chen, Y. P., Liu, X. and Yue, M. (2013). Role and interrelationship of Galpha protein, hydrogen peroxide, and nitric oxide in ultraviolet B-induced stomatal closure in Arabidopsis leaves. Plant Physiol 161(3): 1570-1583.
Category
Plant Science > Plant physiology > Tissue analysis
Plant Science > Plant cell biology > Cell structure
Cell Biology > Cell structure > Cell surface
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










