- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fluorescence Measurement of Postharvest Physiological Deterioration (PPD) in Cassava Storage Roots
Published: Vol 3, Iss 18, Sep 20, 2013 DOI: 10.21769/BioProtoc.909 Views: 12894
Reviewed by: Tie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Simple Sonication Method to Isolate the Chloroplast Lumen in Arabidopsis thaliana
Jingfang Hao and Alizée Malnoë
Aug 5, 2023 2285 Views

A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays
Jason T. Roberts [...] David M. Braun
Oct 20, 2023 2256 Views

Detection and Quantification of Programmed Cell Death in Chlamydomonas reinhardtii: The Example of S-Nitrosoglutathione
Lou Lambert and Antoine Danon
Aug 5, 2024 1589 Views
Abstract
Cassava (Manihot esculenta Crantz) is a perennial root crop in the tropics. Within 24-72 hours of harvest the storage roots deteriorate rapidly, thereby necessitating their prompt processing or consumption. Postharvest physiological deterioration (PPD) of cassava storage roots is the result of a rapid oxidative burst, which leads to discoloration of the vascular tissues. The various fluorogenic probes available for in vivo reactive oxygen species (ROS) imaging could reveal complex spatial and temporal dynamics in plant tissues. Fluorescence measurement of PPD became widely used assay for ROS. Most of the ROS probes passively diffuse across cell membranes localize in the mitochondria, and exhibit fluorescence. Due to its high sensitivity to ROS and ease of loading and detection, the Dihydrorhodamine123 probe has been widely used in plants to monitor ROS accumulation in response to various stimuli and range of developmental processes.
Keywords: CassavaMaterials and Reagents
- DMSO (Sangon Biotech, catalog number: D0231 )
- Dihydrorhodamine123 (Invitrogen, Molecular Probes®, catalog number: D632 ) (see Recipes)
- MitoTracker Deep Red FM (Invitrogen, Molecular Probes®, catalog number: M22426 ) (see Recipes)
- 0.1 M sodium phosphate buffer (pH 7.0) (see Recipes)
Equipment
- 48-well plate
- Razor blade
- Slides
- Centrifuge
- Confocal laser scanning microscope (Zeiss, model: LSM 510 META )
Procedure
- Cut fresh cassava storage roots into smaller segments about 5 x 5 x 0.5 mm in length, width and height by razor blade. The areas (Figure 1):
- The center: vascular tissue found within the center of the root cross-section.
- The middle: the tissue filled with storage cells with streaks of xylem throughout.
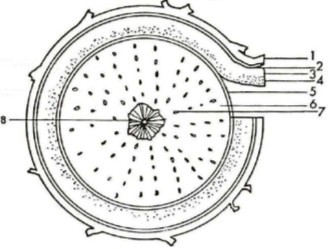
Figure 1. Cassava storage root cross-section (Rickard, 1985). 1: periderm; 2: sclerenchyma; 3: parenchyma; 4: latex tubes; 5: cambium; 6: parenchyma (The middle); 7: xylem vessels (The middle); 8: xylem bundles (The center).
- The center: vascular tissue found within the center of the root cross-section.
- Immersed in the sodium phosphate buffer with Dihydrorhodamine123 or MitoTracker Deep Red FM, stain for 10 and 20 min, respectively in the dark at room temperature.
- Afterwards, wash with sodium phosphate buffer once before viewing under the microscope. Use sodium phosphate buffer to mount the sample on slides.
- Use a Zeiss LSM (Laser Scanning Microscope) 510 META Confocal with the capturing program LSM 510 equipped with a10x and 20x objective.
- Allow the microscope and lasers to warm up for about 20 minutes before use.
- The settings used for each stain are as follows:
- Dihydrorhodamine123 excitation/emission 488/515 nm.
- MitoTracker Deep Red FM excitation/emission 635/680 nm.
- Dihydrorhodamine123 excitation/emission 488/515 nm.
- First use the visible spectrum to find the sample, after that change to fluorescence spectrum (Figure 2).
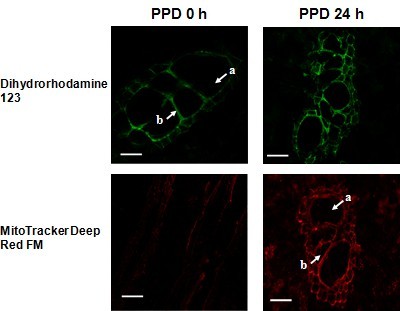
Figure 2. Fluorescence determination of PPD in cassava storage roots. a, xylem vessel; b, bundle sheath; Scale bar = 20 μm.
Recipes
- 0.1 M sodium phosphate buffer, pH 7.0 (100 ml)
Mix 61.5 ml 1 M K2HPO4 and 38.5 ml 1 M KH2PO4
Filter sterilize (0.45 μm)
Store at 4 °C - Dihydrorhodamine123 working solution
- 50 mM Storage solution
10 mg Dihydrorhodamine123 with 577.4 μl DMSO - 50 μM Working solution
Sodium phosphate buffer dilution
Store at -70 °C
- 50 mM Storage solution
- MitoTracker Deep Red FM
- 1 mM Storage solution
50 μg MitoTracker Deep Red FM with 91.98 μl DMSO
- 250 nM Working solution
Sodium phosphate buffer dilution
Store at -70 °C
- 1 mM Storage solution
Acknowledgments
The protocol was mainly adapted from the publication Xu et al. (2013). This work was supported by grants from the National Natural Science Foundation of China (31271775), the National Basic Research Program (2010CB126605), the National High Technology Research and Development Program of China (2012AA101204), the Earmarked Fund for China Agriculture Research System (CARS-12-shzp) and the BioCassava Plus Program from the Bill & Melinda Gates Foundation.
References
- Rickard J. E. (1985). Physiological deterioration in cassava roots. J Sci Food Agric 36: 167-176.
- Xu, J., Duan, X., Yang, J., Beeching, J. R. and Zhang, P. (2013). Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol 161(3): 1517-1528.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Xu, J., Fellman, J. K. and Zhang, P. (2013). Fluorescence Measurement of Postharvest Physiological Deterioration (PPD) in Cassava Storage Roots. Bio-protocol 3(18): e909. DOI: 10.21769/BioProtoc.909.
- Xu, J., Duan, X., Yang, J., Beeching, J. R. and Zhang, P. (2013). Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol 161(3): 1517-1528.
Category
Plant Science > Plant physiology > Abiotic stress
Biochemistry > Other compound > Reactive oxygen species
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












